CBSE Class 11 Chemistry Redox Reactions – Get here the Notes for Class 11 Redox Reactions . Candidates who are ambitious to qualify the Class 11 with good score can check this article for Notes. This is possible only when you have the best CBSE Class 11 Chemistry study material and a smart preparation plan. To assist you with that, we are here with notes. Hope these notes will helps you understand the important topics and remember the key points for exam point of view. Below we provided the Notes of CBSE Class 11 Chemistry for topic Redox Reactions.
- Class: 11th
- Subject: Chemistry
- Topic: Redox Reactions
- Resource: Notes
CBSE Notes Class 11 Chemistry Redox Reactions
Candidates who are pursuing in CBSE Class 11 Chemistry are advised to revise the notes from this post. With the help of Notes, candidates can plan their Strategy for particular weaker section of the subject and study hard. So, go ahead and check the Important Notes for CBSE Class 11 Chemistry Redox Reactions from this article.
Chemical reactions which involves both oxidation as well as reduction process simultaneously, are known as redox reactions (‘red’) from reduction and ‘ox’ from oxidation). All these reactions are always accompanied by energy change in the form of heat, light or electricity.
Types of Redox Reactions
(i) Intermolecular redox reactions In such reactions, oxidation and reduction take place separately in two compounds. e.g.,
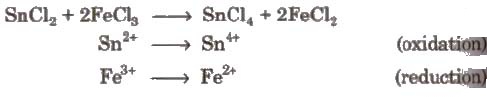
(ii) Intramolecular redox reactions In these reactions, oxidation and reduction take place in a single compound. e.g.,
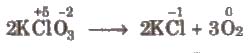
(iii) Disproportionation reactions These reactions involve reduction and oxidation of same element of a compound. e.g.,
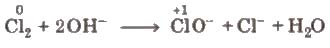
This reaction is also known as autoredox reaction.
Classification of Redox Reactions
1. Direct Redox Reactions
Chemical reaction in which oxidation as well as reduction is carried out simultaneously in the same container, is known as direct redox reaction In such reactions, energy is generally liberated in the form of heat energy.
2. Indirect Redox Reactions
A reaction in which oxidation and reduction are carried out separately in two separate half-cells, is known as indirect redox reaction. In such reactions, energy is generally liberated in the form of electrical energy.
oxidation and Reduction

Reductants and Oxidants
Oxidant or oxidising agent is a chemical substance which can accept one or more electrons and causes oxidation of some other species. In other words, the oxidation number of oxidant decreases in a redox reaction.
Important Oxidants
Molecules of most electronegative elements such as O2, O3, halogens.
Compounds having element in its highest oxidation state e.g.,
K2Cr2O7, KMnO4, HCIO4, H2SO4, KCIO3, Ce(SO4)2,
Oxides of metals and non-metals such as MgO, CrO3, CO2, etc.
Reductant or reducing agent is a chemical.substance which can give one or more electrons and causes reduction of some other species. In other words, the oxidation number of reductant increases in a redox reaction.
Important Reductants
All metals such as Na, AI, Zn, etc., and some non – metals, e.g., C, S. P, H2, etc.
Metallic hydrides like NaH, LiH. KH, CaH2 etc.
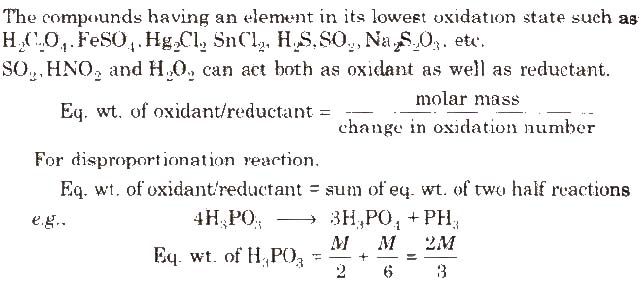
Oxidation Number
The oxidation number is defined as the charge in which an atom appears to have when all other atoms are removed from it as ions. It may have + or – sign.
[An element may have different values of oxidation number depending upon the nature of compound in which it is present.]
Oxidation number of an element may be a whole number (positive or negative) or fractional or zero.
Important Points for Determining Oxidation Number
- The algebraic sum of the oxidation numbers of aU the atoms in an uncharged (neutral) compound is zero. In an ion, the algebraic sum is equal to the charge on the ion.
- All elements in the elementary state have oxidation number zero, e.g., He, Cl2, S8, P4 etc.
- As fluorine is the most electronegative element, it always has an oxidation number of – 1 in all of its compounds.
- In compounds containing oxygen, the oxidation number of oxygen is – 2 except in peroxides (-1) such as Na2O2, in OF2 and in O2 F2 (+2 and + 1 respectively).
- In all compounds. except ionic metallic hydrides, the oxidation number of hydrogen is +1. In metal hydrides like NaH, MgH2, CaH2, LiH, etc the oxidation number of hydrogen is -1.
- Oxidation number for alkali metals is +1 and for alkaline earth metals is + 2.
- Oxidation number of metal in amalgams is zero.
- In case of coordinate bond, it gives +2 value of oxidation number to less electronegative atom and -2 values to more electronegative atom when coordinate bond is directed formless electronegative atom to more electronegative atom .
- If coordinate bond is directed from more electronegative to less electronegative atom then its contribution be zero for both the atoms.
- For p-block elements [Except F and 0], the highest oxidation number is equal to their group number and lowest oxidation number is equal to the group number minus eight.
- In transition elements the lowest oxidation number is equal to the number of ns electrons and highest oxidation number is equal to number of ‘ns’ and (n – l)d unpaired electrons.
Determination of Oxidation Number of Underlined Element
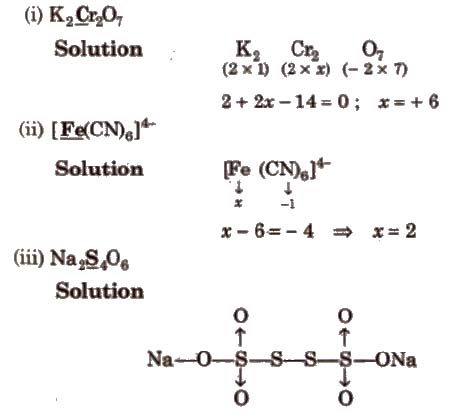
Oxidation number of Na = + 1
Oxidation number of 0 = – 2
∴ 2 (1) + 4x + 6 x – 2 = 0
a = 5 / 2, this is average oxidation number. because the compound has two types of sulphur atom.
OX of sulphur bonded with coordinate bond = 5
ON of sulphur which have S-S bond = 0
Average oxidation number = 5 + 5 + 0 + 0 / 4 = 5 / 2
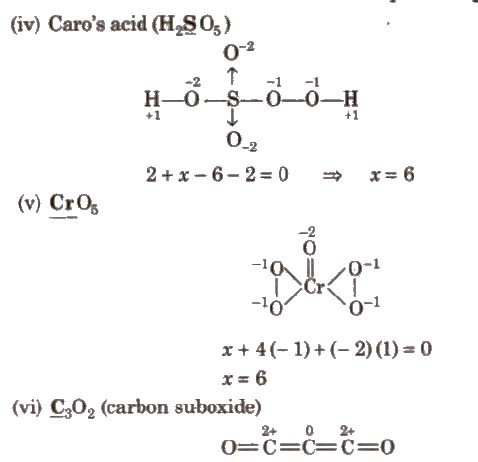
(vii) NH4 NO3
There are two types of nitrogen atoms. Therefore. evaluation should be made separately as
Oxidation number of N in NH+4
x + 4 (+ 1)= + 1
x = – 3
Oxidation number of N in NO–3
y + 3 x (- 2) = – 1
y = 5
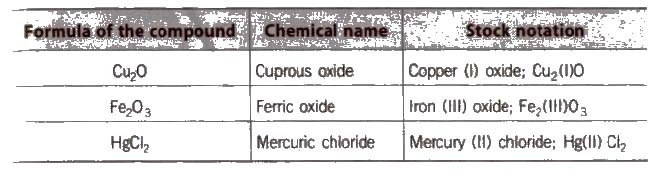
Stock Notations
The oxidation states of elements exhibiting variable oxidation states are specified by Roman numerals such as I, II, III, IV, etc., within parenthesis after the symbol or name of the element. This system was introduced for the first time by German chemist, Alfred Stock and is known as Stock notation. This may be illustrated as
Balancing of Redox Chemical Equations
Every chemical equation must be balanced according to law of conservation of mass. In a balanced chemical equation the atoms of various species involved in the reactants and products must be equal in number. Redox reaction can be balanced through (i) Ion electron method (ii) Oxidation number method
Ion Electron Method
This method of balancing was developed by Jette and Lamer in 1927.
For example. balance the equation
Cu + HNO3 → Cu(NO3)2 + NO + H2O
It involves the following steps.
Step I Write the redox reaction in ionic form
Cu + H+ + NO–3 → Cu2+ + NO + H2O
Step II Split the redox reaction into its oxidation-half and reduction half-reaction.

Step III Balance atoms of each half-reaction (except H and O) by using simple multiples.
Cu → Cu2+ and NO–3 → NO
(Except H and O, all atoms are balanced)
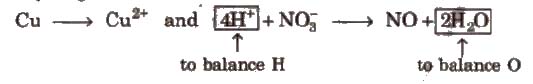
Step IV Balance H and O as
(i) For acidic and neutral solutions Add H2O molecule to the side deficient in oxygen and H+ to the side deficient in hydrogen.
(ii) For alkaline solutions For each excess of oxygen, add one water molecule to the same side and OH– ion to the other side to balance H.
Step V Add electrons to the side deficient in electrons.
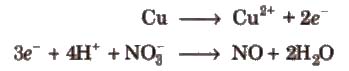
Step VI Equalize the number of electrons in both the reactions by multiplying a suitable number
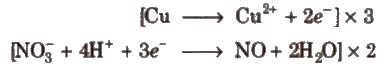
Step VII Add the two balanced half reactions and cancel common terms of opposite sides
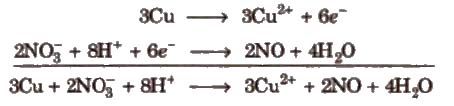
Step VIII Convert the ionic reaction into molecular form by adding spectator ions
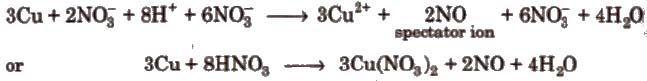
(Ions which are present in solution but do not take part in the redox reaction, are omitted while writing the net ionic equation of a reaction and are known as spectator ions.)
Oxidation Number Method
For example, balance the equation
Mg + HNO3 → Mg(NO3)2 + N2O + H2O
It involves the following steps.
Step I Write the skeleton equation (if not given)
Step II Assign oxidation number of each atom
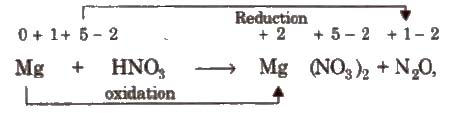
Step III Balance atoms other than H and O in two processes.
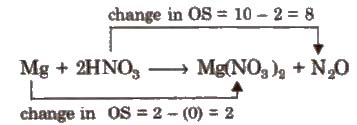
Step IV Equalize the total increase or decrease in oxidation number
4Mg + 2HNO3 → 4Mg(NO3)2 + NO2O
Step V Balance H and O
8H+ + 4 Mg + 2HNO3 + 8NO3– → 4 Mg (NO3)2 + N2O + 5H2O
4 Mg + 10 HNO3 → 4 Mg (NO3)2 + N2O + 5H2O
Redox Reactions in Daily Life
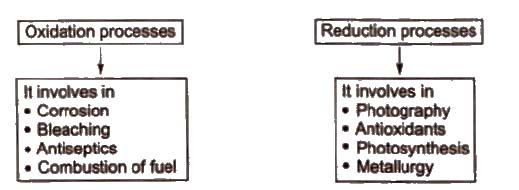
Class 11 Key Points, Important Questions & Practice Papers
Hope these notes helped you in your schools exam preparation. Candidates can also check out the Key Points, Important Questions & Practice Papers for various Subjects for Class 11 in both Hindi and English language form the link below.
Class 11 NCERT Solutions
Candidates who are studying in Class 11 can also check Class 11 NCERT Solutions from here. This will help the candidates to know the solutions for all subjects covered in Class 11th. Candidates can click on the subject wise link to get the same. Class 11 Chapter-wise, detailed solutions to the questions of the NCERT textbooks are provided with the objective of helping students compare their answers with the sample answers.
Class 11 Mock Test / Practice
Mock test are the practice test or you can say the blue print of the main exam. Before appearing in the main examination, candidates must try mock test as it helps the students learn from their mistakes. With the help of Class 11 Mock Test / Practice, candidates can also get an idea about the pattern and marking scheme of that examination. For the sake of the candidates we are providing Class 11 Mock Test / Practice links below.
Class 11 Exemplar Questions
Exemplar Questions Class 11 is a very important resource for students preparing for the Examination. Here we have provided Exemplar Problems Solutions along with NCERT Exemplar Problems Class 11. Question from very important topics is covered by Exemplar Questions for Class 11.
CBSE Notes for Class 11 Chemistry Maths Notes Physics Notes Biology Notes
To get study material, exam alerts and news, join our Whatsapp Channel.

