Class 12 Chemistry Polymers – Get here the Notes for Class 12 Polymers. Candidates who are ambitious to qualify the Class 12 with good score can check this article for Notes. This is possible only when you have the best CBSE Class 12 Chemistry Notes, study material, and a smart preparation plan. CBSE 2019 Class 12th Exam is approaching and candidates will have to make the best use of the time available towards the last stage of your CBSE Class 12th Chemistry Preparation. To help you with that, below we have provided the Notes of 12 Chemistry for topic Polymers.
- Class: 12th
- Subject: Chemistry
- Topic: Polymers
- Resource: Notes
CBSE Notes Class 12 Chemistry Polymers
Candidates who are pursuing in Class 12 are advised to revise the notes from this post. With the help of Notes, candidates can plan their Strategy for particular weaker section of the subject and study hard. So, go ahead and check the Important Notes for Class 12 Chemistry Polymers.
The word polymer has a Greek origin. which means many units (parts). Polymer is defined as a chemical substance of a high molecular mass formed by the combination of a large number of simple molecules, called monomers. e.g.,
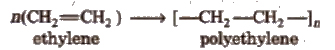
Polymerisation
The process by which the monomers get combined and transformed into polymers. is known as polymerisation.
n [Monomer] → Polymer
Difference between Polymers and Macromolecules
Polymers are also called macromolecules due to their large size but converse is not always true. A macromolecule mayor may not contain monomer units, e.g., chlorophyll (C55H72O5N4Mg) is a macromolecule but not a polymer since there are no monomer units present so we can conclude that all polymers are macromolecules while all macromolecules may not be polymers in nature.
Classification of Polymers Based on Source of Origin
(i) Natural polymers Those polymers which occur in nature. i.e., in plants or animals. are called natural polymers.

(ii) Synthetic polymers The polymers which are prepared in the laboratory are known as synthetic polymers or man-made polymers, e.g., polythene, synthetic rubber, PVC, nylon-66, teflon, orlon etc.
(iii) Semisynthetic polymers Polymers obtained by making some modification in natural polymers by artificial means, are known as semi synthetic polymers, e.g., cellulose acetate (rayon), vulcanised rubber etc.
Classification of Polymers Based on Structure

(i) Linear polymers These are the polymers in which the monomer units are linked to one another to form long linear chains. These linear chains are closely packed in space. The close packing results in high densities, tensile strength and high melting and boiling points. e.g., high density polyethene, nylon and polyesters are linear polymers.

(ii) Branched chain polymers In such polymers, the monomer units are linked to form long chains with some branched chains of different lengths with source. As a result of branching, these polymers are not closely packed in space. Thus, they have low densities, low tensile strength as well as low melting and boiling points. Some common examples of such polymers are low density polyethene, starch, glycogen etc.
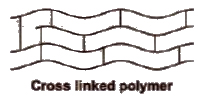
(iii) Cross-linked polymers or network polymers In such polymers, the monomer units are linked together to form three dimensional network. These are expected to be quite hard, rigid and brittle. Examples of cross linked polymers are bakelite, glyptal, melamine-formaldehyde polymer etc.
Classification of Polymers Based on Mode of Polymerisation
(i) Addition polymers The polymers formed by the polymerisation of monomers containing double or triple bonds (unsaturated compounds) are called addition polymers. Addition polymers have the same empirical formula as their monomers.
Addition polymers can further be classified on the basis of the types
of monomers into the following two classes:
Homopolymers The polymers which are obtained by the polymerisation of a single type of monomer are called homopolymers.
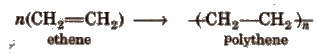
Copolymers The polymers which are obtained by the polymerisation of two or more monomers are called copolymers
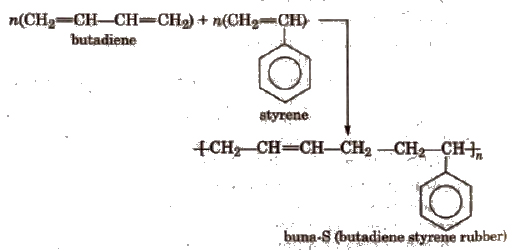
(ii) Condensation polymers The polymers which are formed by the combination of monomers with the elimination of small molecules such as water, alcohol, hydrogen chloride etc., are known as condensation polymers, e.g., nylon 6,6 is formed by the condensation of hexamethylene diamine with adipic acid.
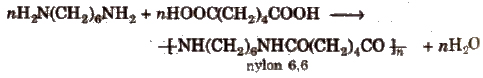
Classification of Polymers Based on Molecular Forces
- Elastomers These are rubber like solid polymers in which the polymer chains are held together by weakest intermolecular forces, e.g., natural rubber, buna-S, buna-N etc . The weak binding forces permit the polymers to be stretched. A few ‘cross links’ are introduced in between the chains, which help the polymer to retract to its original position after the force is released as in vulcanised rubber.
- Fibres Fibres belong to a class of polymers which are thread-like and can be woven into fabrics. These are widely used for making clothes, nets, ropes, gauzes, etc. Fibres possess high tensile strength because the chains possess strong intermolecular forces such as hydrogen bonding. The fibres are crystalline in nature and have sharp melting points. A few examples of this class are nylon-66, terylene and polyacrylonitrile.
- Thermoplastics These are linear polymers and have weak van der Waals’ forces acting in the various chains. These forces are intermediate of the forces present in the elastomers and in the fibres. When heated, they melt and form a fluid which sets into a hard mass on cooling. Thus, they can be cast into different shapes by using suitable moulds, e.g., polyethene and polystyrene.
(Plasticizers are high boiling esters or haloalkanes. These are added to I plastics to make them soft rubber like. …J - Thermosetting plastics These are normally semifluid substances with low molecular masses. When heated, they become hard and infusible due to the cross-linking between the polymer chains. As a result, they also become three dimensional in nature. A few common thermosetting polymers are bakelite, melamine-formaldehyde resin and urea formaldehyde resin.
Types of Polymerisation
1. Chain Growth Polymerisation or Addition Polymerisation
It involves formation of reactive intermediate such as free radical, a carbocation or a carbanion. For this polymerisation monomers used are unsaturated compounds like alkenes; alkadienes and their derivatives. Depending upon the nature of the reactive species involved. chain growth polymerisation occurs by the following mechanisms:
- Free radical addition polymerisation
- Cationic polymerisation
- Anionic polymerisation
(i) Free radical addition polymerisation The monomers used are generally monosubstituted alkenes. The most commonly used catalysts are benzoyl peroxide, hydrogen peroxide or t-butyl peroxide etc.
Mechanism The reaction involves the following steps
Step I Chain initiation step In this step, peroxide undergoes homolytic fission, e.g., benzoyl peroxide on heating produces phenyl initiator free radical.
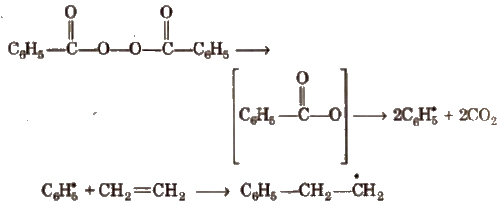
Step II Chain propagation step The new free radical adds to another molecules of monomer to form a larger free radical.
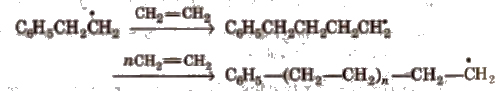
Step III Chain termination step There are three ways of chain termination: Coupling reaction, disproportionation reaction, chain transfer reaction. One mode of termination of chain is shown as under:
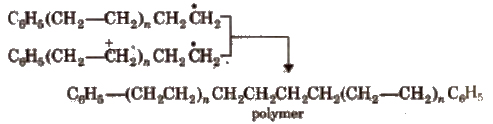
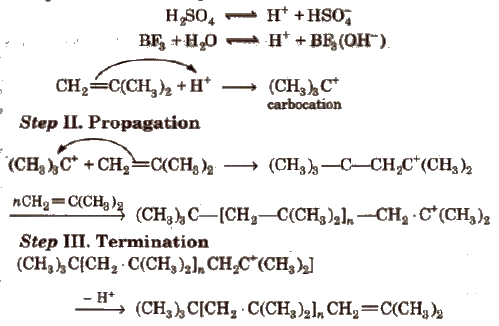
(ii) Cationic polymerisation It involves formation of carbocation which are generated by Lewis acids (like BF3, AICI3, SnCI4, etc.) and protonic acids such as H2SO4, HF, etc.
Higher the stability of carbocation intermediate, more is the reactivity of monomers towards cationic addition polymerisation. It involves the following steps:
Step I. Initiation Step
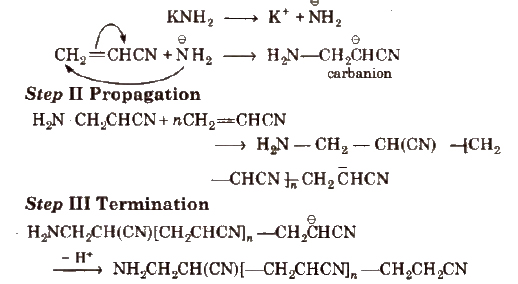
(iii) Anionic polymerisation It involves formation of a carbanion, Steps involved in this process are
Step I Initiation Strong bases act as initiator.
Step Growth Polymerisation
Condensation polymerisation which occurs in a stepwise manner with elimination of some smaller molecules like H2O, NH3, HCI, ROH, etc., is concerned with step growth polymerisation, e.g., adipic acid and hexamethylenediamine phenol and formaldehyde etc., undergo step Growth Polymerisation.
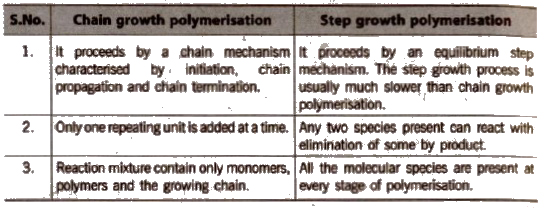
Distinction Between Chain Growth Polymerisation and Step Growth Polymerisation
Molecular Mass of Polymers
The growth of the polymer chain depends upon the availability of the monomers in the reaction. Thus, the polymer sample contains chain of varying lengths and hence, its molecular mass is always expressed as an average molecular mass.
Number-Average Molecular Mass Mn
If N1 molecules have molecular mass M1 each, N2 molecules have molecular mass M2 each, N3 molecules have molecular mass M3 each and so on,
then, Mn = Σ Ni Mi / Σ Ni
It is determined by osmotic pressure method.
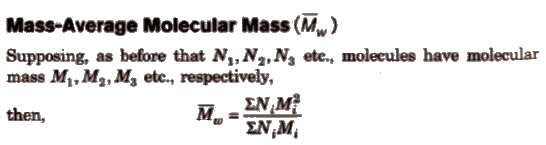
It is determined by light scattering and ultracentrifugation method.
PoLydispersity Index
It is the ratio of the mass average molecular mass to the number average molecular mass
PDI = Mw / Mn
For natural polymers, PDI is usually equal to one which means that they are monodisperse. In other words, such polymers are more homogeneous. On the contrary, synthetic polymers generally have PDI > 1 which means that they are less homogeneous.
Polyolefins
These are obtained by the addition polymerisation of ethylene and its derivatives
1. Polythene
Polymer of ethylene or ethene.
(i) Low density polythene (LDP)
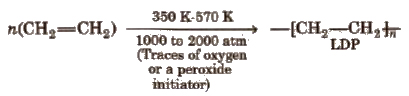
It is tough, flexible, transparent, chemically inert as well as poor conductor pf electricity. It has moderate tensile strength but good tearing strength.
It is used in the insulation of electricity carrying wires and manufacture of queeze bottles, toyes and flexible pipes.
(ii) High density polyethylene (HOP)
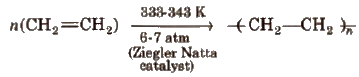
It has high density due to close packing. It is also chemically inert and more tougher and harder.
It is used for making containers, house wares, bottles, toyes, electric insulation etc.
2. Polystyrene (Styrone)
The monomers are styrene molecules. It is thermoplastic. It is used for making toys, radio and TV cabinets
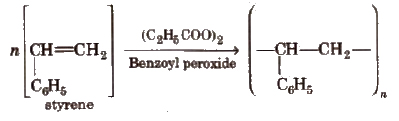
3. Polyvinylchloride (PVC)
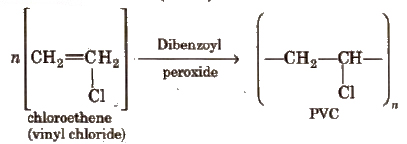
It is used for making rain coats, toys, electrical insulation. It is hard and resistant to heat and chemicals.
4. Polypropylene (PP)
It is obtained by polymerising propylene in the presence of Ziegler-Natta catalyst.
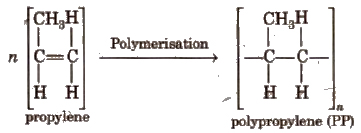

It is chemically inert and resistant to attack by corrosive reagent. It is used in making oil seals, gaskets and also for non-stick surface coated utensils.
6. Polyacrylonitrile
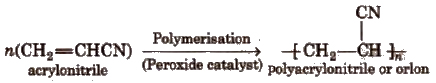
It is used as a substitute for wool in making commercial fibres as orIon or acrilan.
Polyamides
The polymers which contain an amide linkage in chain are known as pOlyamide, e.g., nylon-6, 6.
1. Nylon-66
It is obtained by the condensation of adipic acid and hexamethylenediamine with the elimination of water molecule
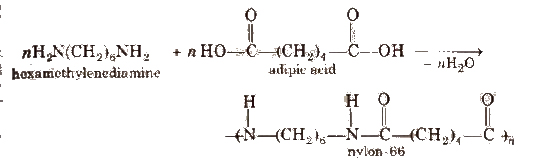
The polyamides are identified by numbers. These numbers refer to the number of carbon atoms in diamine and in the dibasic acid. As in the above case, the carbon atoms are 6 in each case, therefore the product is described as _nylon-66.
Properties and uses
Nylon-66 is a linear polymer and has very high tensile strength. It shows good resistance to abrasion. Nylon-66 is usually fabricated into sheets. It is used in bristles for brushes and in textile
2. Nylon-6
Nylon-6 is obtained by heating caprolactam with water at a high temperature.
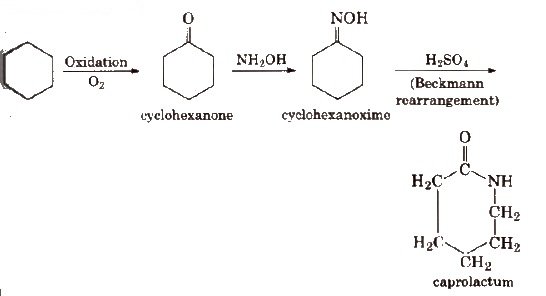
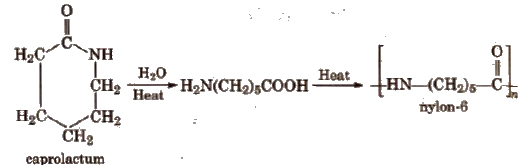
Resins
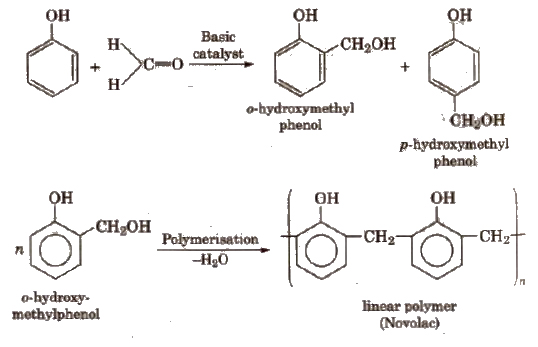
1. Phenol-Formaldehyde Polymer
(Bakelite and Related Polymers)
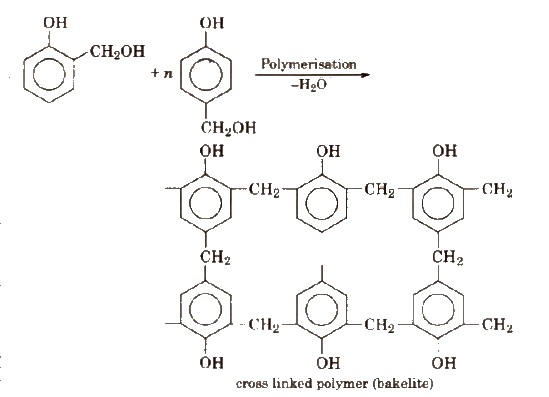
These polymers are obtained by the condensation reaction of phenol with formaldehyde in the presence of either acid or a base catalyst. The reaction involves the formation of methylene bridge at ortho, para or both ortho and para positions. A linear or cross linked material is obtained depending upon the condition of reaction.
Uses
Bakelite is used for making combs, photograph records, electrical switches etc. Soft bakelites with low degree of polymerisation are used as binding glue for laminated wooden plants, in varnishes and lacquers.
2. Melamine-formaldehyde Resin
It is a copolymer formed by the polymerisation of melamine (which is a heterocyclic triamine) and formaldehyde as follows :
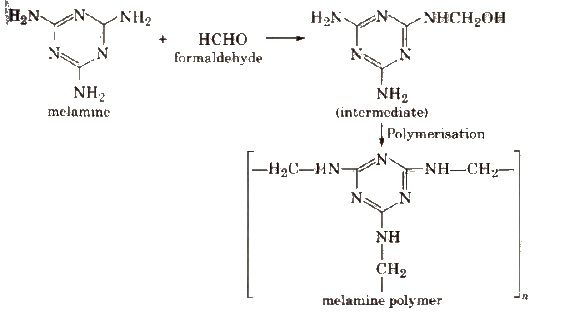
Properties and Uses
It is very hard and tough. It has assumed great importance these days particularly in making crockery. They do not break even when droped from a height.
3. Urea-formaldehyde Resin
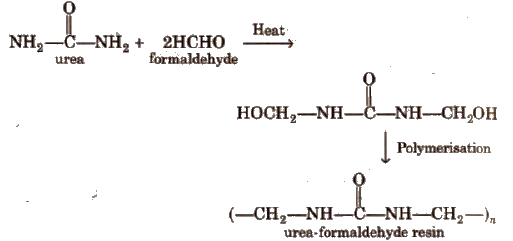
4. Natural Rubber
Natural.rubber is a coiled linear 1, 4-polymer of isoprene.
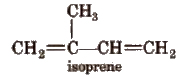
In the polymer chain of natural rubber, the residual double bonds are located between C2 and C3 of the isoprene unit. All these double bonds have cis configuration, and thus natural rubber is cis-l,4-polyisoprene.
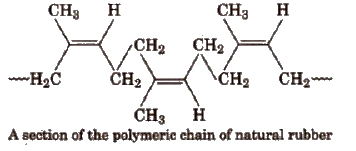
In the natural rubber, there is no polar substituent. The only intermolecular forces are van der Waals’ type. The cis-configuration gives the polymeric chain of natural rubber a coiled structure. As a result, it can be stretched by the application of a force. When the force is removed, the chain returns back to its original coiled shape.
Natural rubber is soft and sticky. It can be used only in the temperature range 10°C-50°C. At higher temperature, it becomes soft and at low temperature, it becomes brittle. It has higb water absorption capacity. It is attacked by oxidising agents and organic solvents. As such, it cannot be used very extensively for commercial puposes.
Vulcanisation of Rubber
The properties of natural rubber can be modified by introducing -S-S- polysulphide crosslinks in its structure. This process of introducing -S-S- crosslnks in the structure of natural rubber by heating with sulphur at 11O°C is called vulcanlsation of rubber.
Vulcanisatlon is carried out by adding sulphur (3-5%) and zinc oxide to the rubber, and then heating the object at about 110°Cfor about 20-30 minutes. Zinc oxide accelerates the rate of vulcanisation. Vulcanisation introduces polysulphide (-S-S-) bonds between the adjacent chains. These crosslinks tend to limit the motion of chains relative to each other.
5. Neoprene
Polymer formed by polymerisation of chloroprene is neoprene or synthetic rubber.
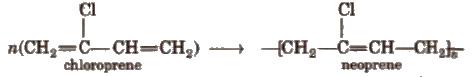
It is used for the manufacturing conveyers belts, gasket and hoses.
6. Buna-N
It is a copolymer of buta-I, 3-diene and acrylonitrile. It is formed as follows
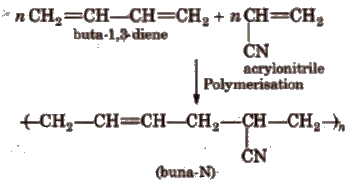
Properties and Uses
It is insulator in nature and is used for making conveyor belts and printing rollers.
Polyesters
The polymers which contain an ester linkage are known as polyester, e.g., dacron.
1. Polymethylmethacrylate (PMMA)
It is prepared by the polymerisation of methylmethacrylate in the presence of suitable organic peroxide.

The polymer is known by several commercial names such as lucite, acrylite, plexiglass and perspex.
Properties and uses
It is a hard and transparent polymer and is quite resistant to the effect of light, heat and ageing. It is used, in the manufacture of unbreakable lights, protective coatings, dentures, and in making windows for aircrafts.
2. Glyptal
It is a polyester having crosslinks. It is a thermosetting plastic. It is obtained by condensation of ethylene glycol and phthalic acid or glycerol and phthalic acid.
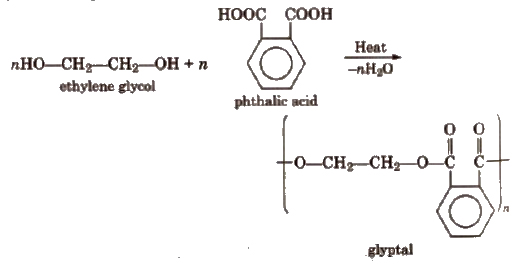
When its solution in a suitable solvent is evaporated, it leaves a tough but non-flexible film. It is, therefore, used in the manufacture of paints and lacquers.
3. Terylene (Dacron)
It is a condensation product of ethylene glycol and terephthalic acid.
Polymerisation is carried out at 420 to 460 K in the presence of catalyst mixture of zinc acetate and antimony trioxide.
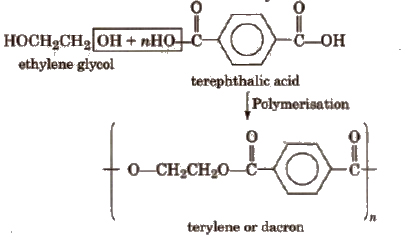
Properties and uses
Terylene is highly resistant to the action of chemical and biological agents. Its fibres are quite strong and durable. It can also be blended with wool or cotton to obtain fabrics of desired composition.
Terylene is used in the manufacture of a variety of clothes such as terycot, terywool and terysilk as a result of blending with other yerns. It is also used for preparing magnetic recording tapes, conveyer belts, aprons for industrial workers etc.
Biopolymers and Biodegradable Polymers
Synthetic polymers are mostly non-biodegradable i.e., it is very difficult to dispose off the polymeric waste, e.g., polythene bags.
Nature has provided us a variety of polymers which can be produced by the biological systems in plants and animals. These are called biopolymers, e.g., polysaccharides, proteins, nucleic acids, etc. In the biological system, these polymers decompose or hydrolyse in the Presence of different enzymes. This means that they are biodegradable.
Aliphatic polyesters are the common examples of biodegradable Polymers.
It is a copolymer of 3-hydroxybutanoic acid and 3-hydroxypentanoic acid.
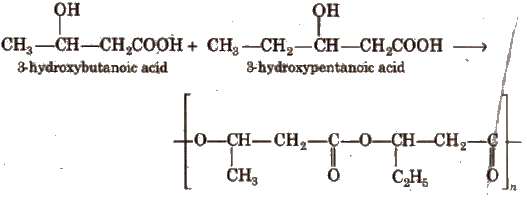
2. Nylon-2-Nylon-6
It is an alternating polyamide copolymer of glycine (H2N-CH2-COOH) and amino caproic acid [H2N(CH2) 5COOH] and is biodegradable.
Some More Impotant Polymers
- Saran is a copolymer of vinyl chloride and Isused for wrapping food materials.
- ASS rubber is a copolymer of acrylonitrile, buta-1, 3-diene and styrene.
- Bubble gum contains styrene butadiene rubber.
- Epoxy resins are used In making adhesives such as araldite, etc. These are the copolymer of epichlorohydrin and bisphenol-A.
- Thikol is another variety of synthetic rubber which is a copolymer of ethylene chloride and sodium tetrasulphide (Na2S44).
- Dynells a copolymer of vinyl chloride and acrylonitrile and is used for making human hair wigs.
- Silk Is a thread like natural polymer which is obtained from cocoons of sllk worms. It is a natural polyamide fibre.
- Thermocol Is a foamed plastic obtained by blowing air through molter polystyrene or polyurethane.
- Superglue is a polymer of methyl α-cyanoacrylate and is obtained by anionic polymerisation of monomer.
Class 12 Key Points, Important Questions & Practice Papers
Hope these notes helped you in your schools exam preparation. Candidates can also check out the Key Points, Important Questions & Practice Papers for various Subjects for Class 12 in both Hindi and English language form the link below.
Class 12 NCERT Solutions
Candidates who are studying in Class 12 can also check Class 12 NCERT Solutions from here. This will help the candidates to know the solutions for all subjects covered in Class 12th. Candidates can click on the subject wise link to get the same. Class 12 Chapter-wise, detailed solutions to the questions of the NCERT textbooks are provided with the objective of helping students compare their answers with the sample answers.
Class 12 Mock Test / Practice
Mock test are the practice test or you can say the blue print of the main exam. Before appearing in the main examination, candidates must try mock test as it helps the students learn from their mistakes. With the help of Class 12 Mock Test / Practice, candidates can also get an idea about the pattern and marking scheme of that examination. For the sake of the candidates we are providing Class 12 Mock Test / Practice links below.
Class 12 Exemplar Questions
Exemplar Questions Class 12 is a very important resource for students preparing for the Examination. Here we have provided Exemplar Problems Solutions along with NCERT Exemplar Problems Class 12. Question from very important topics is covered by Exemplar Questions for Class 12.
Class 12 Chemistry Maths Notes Physics Notes Biology Notes
To get study material, exam alerts and news, join our Whatsapp Channel.

