Class 12 Chemistry Amines – Get here the Notes for Class 12 Amines. Candidates who are ambitious to qualify the Class 12 with good score can check this article for Notes. This is possible only when you have the best CBSE Class 12 Chemistry Notes, study material, and a smart preparation plan. CBSE 2019 Class 12th Exam is approaching and candidates will have to make the best use of the time available towards the last stage of your CBSE Class 12th Chemistry Preparation. To help you with that, below we have provided the Notes of 12 Chemistry for topic Amines.
- Class: 12th
- Subject: Chemistry
- Topic: Amines
- Resource: Notes
CBSE Notes Class 12 Chemistry Amines
Candidates who are pursuing in Class 12 are advised to revise the notes from this post. With the help of Notes, candidates can plan their Strategy for particular weaker section of the subject and study hard. So, go ahead and check the Important Notes for Class 12 Chemistry Amines.
Amines constitute an important class of organic compounds derived by replacing one or more hydrogen atoms ofNH 3 molecule by alkyl/aryl group(s).
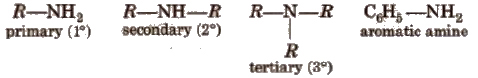
In the IUPAC system, the amines are regarded as alkanamines, e.g.,
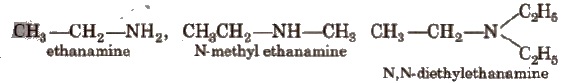
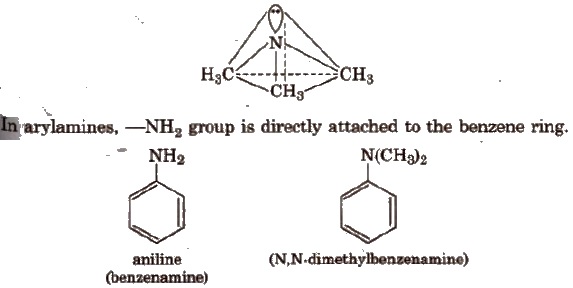
Structure
The nitrogen atom in amine is spa-hybridised. The three hybrid orbitals are involved in bond formation and one hybrid atomic orbital contains the lone pair of electrons, giving the pyramidal geometry of amines.
Methods of Preparation of Amines
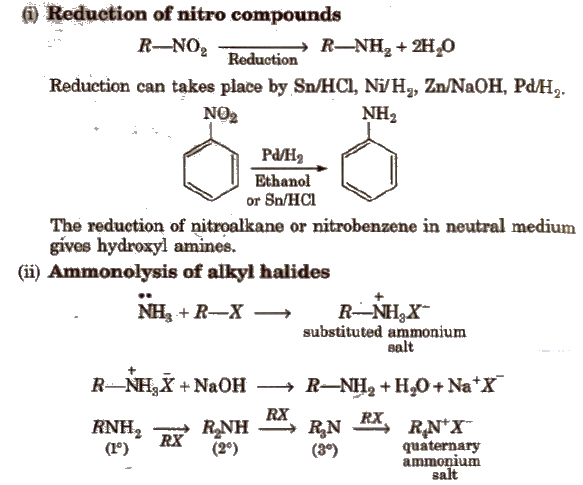
Ammonolysis has the disadvantage of yielding a mixture of primary, secondary and tertiary amines and also a quaternary ammonium salt.
However, primary amine is obtained as a major product by taking large excess of NH3.
Order of reactivity of halides ‘with amines is RI > RBr > RCI.
Aromatic amines could not be prepared since aryl halides are much less reactive towards nucleophilic substitution reactions.
(iii) Reduction of nitriles or cyanides
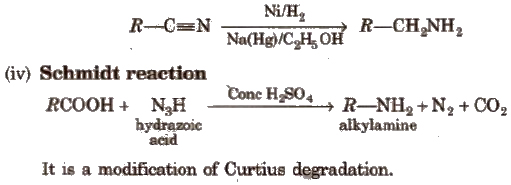
(v) Reduction of amides
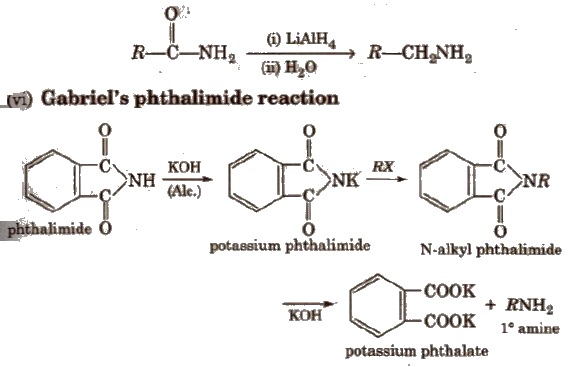
It only produces 1 0 amines. This method is not suitable for 1° arylamine because aryl halide does not give nucleophilic substitution reaction.
(viii) Hofmann bromamide degradation reaction
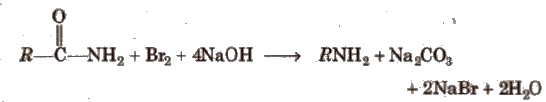
In Hofmann degradation reaction, the amine formed has one carbon less than the parent amide. To obtain primary amine with same number of carbon atoms from primary amide, reduction is done with LiAlH4/ether.
Physical Properties of Amines
- The lower aliphatic amines are gases with fishy smell.
- Primary amines witb three or more carbon atoms are liquid and higher members are all solids.
- Lower aliphatic amines are water suluble because they can form hydrogen bonds with water molecules, however the solubility decreases with increase in hydrophobic alkyl group.
- Boiling points order primary > secondary > tertiary
- Tertiary amines does not have intermolecular association due to the absence of hydrogen atom available for hydrogen bond formation.
Basic Strength of Amines
Amines act as Lewis bases due to the presence of lone pair of electrons on the nitrogen atom.
More the Kb (dissociation constant of base), higher is the basicity of amines.
Lesser the pKb‘ higher is the basicity of amines.
Aliphatic amines (CH3NH2) are stronger bases than NH3 due to the electron releasing +/ effect of the alkyl group.
Among aliphatic methyl amines, the order of basic strength in aqueous solution is as follows
(C2H5NH > (C2H5)3N > C2H5NH2 > NH3
(CH3)2NH > CH3NH2 > (CH3)3N > NH3
Aromatic amines are weaker basesthan aliphatic amlnes and NH3,due to the fact that the electron pair on the nitrogen atom is involved in resonance with the π-electron pairs of the ring.
Electron releasing groups (e.g.,-CH3,-OCH3,-NH2 etc.) increase the basic strength of aromatic amines while electron withdrawing groups (like – NO2, -X,-CN etc.) tend to decrease the same.
o-substituted aromaticamines are usually weaker basesthan aniline irrespective of the nature of substituent whether electron releasing or electron withdrawing. This is called ortho effect and is probably due to sterk and electronic factors.
chemical Properties of Amines
(i) Alkylation All the three types of amines react with alkyl halides to form quaternary ammonium salt as the final product provided alkyl halide is present in excess.
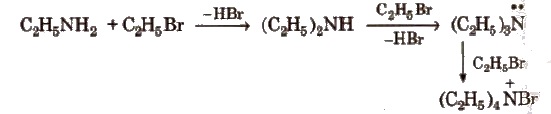
Aromatic amines also undergo alkylation as given below.
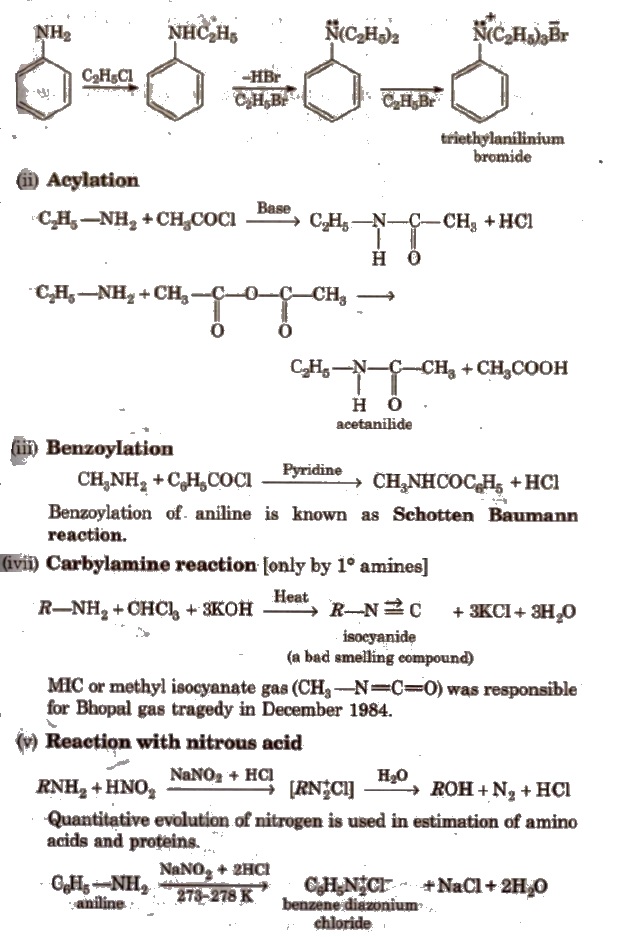
But secondary and _tertiary amines react with nitrous acid in different manner.
Methyl amine give dimethyl ether with HNO2.
(vi) Reaction with aryl sulphonyl chloride [Hinsberg reagent] The reaction of benzenesulphonyl chloride with primary amine yield N-ethyl benzenesulphonyl amide.
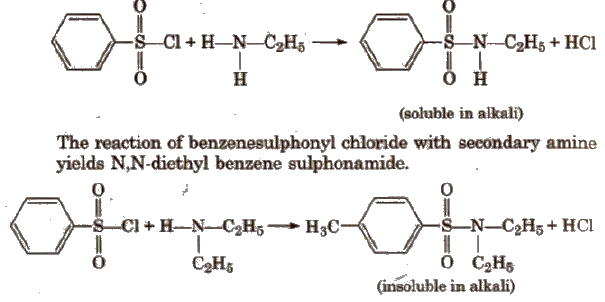
Tertiary amines does not react with benzenesulphonyl chloride.
(vii) Reaction with aldehydes Schiff base is obtained.
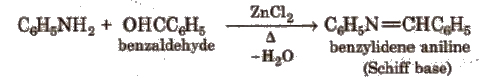
(viii) Electrophihc substitution reactions Aniline is ortho and para directing towards electrophilic substitution reaction due to high electron density at ortho and para-positions.
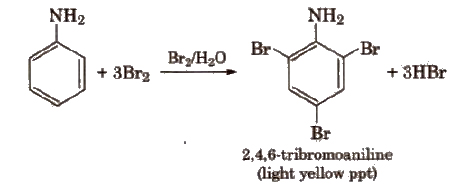
To prepare monosubstituted derivative, activating effect of -NH2 group must be controlled. It can be done by protecting the -NH2 group by acetylation with acetic anhydride.

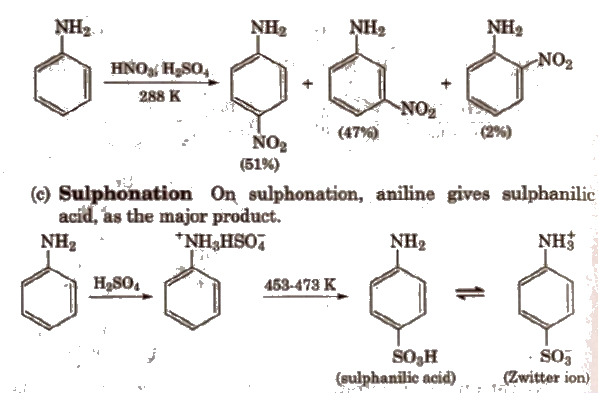
(b) Nitration Direct nitration of aniline is not possible as it is susceptible to oxidation, thus amino group is first protected by acetylation.
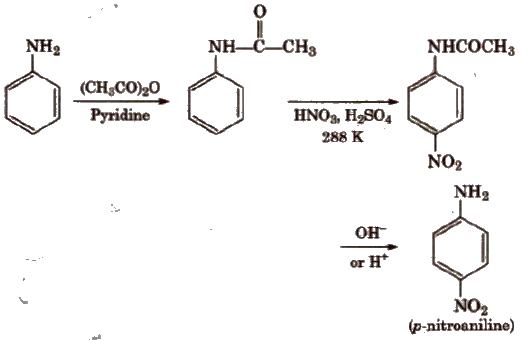
In strongly acidic medium, aniline is protonated as anilinium ion which is meta directing so it gives meta product also.
(d) Aniline does not undergo Friedel-Crafts reaction due to salt formation with aluminium chloride, the Lewis acid, which is used as a catalyst. Due to this, nitrogen of aniline acquires positive charge and hence. behave like a strong deactivating group for further chemical reaction.
(ix) Oxidation Use of diffrent oxidising agents gives difTerent products.
e.g.,

Separation of Mixture of Amines (1°, 2° and 3°)
(a) Fractional distillation This method Is based on the boiling points of amines and is used satIsfactorily in Industry.
(b) Hofmann’s methoOd Diethyloxalate is called Hofmann’s reagent with which mixture of amines is treated.
- 1° amine forms solid dialkyl oxamide (CONHR)2
- 2° amine forms liquid dialkyl oxamlc ester(CONR2-COOC2H5)
- 3° amlnes do not react
(c) Hlnsberg’s method see.chemkal reactions.
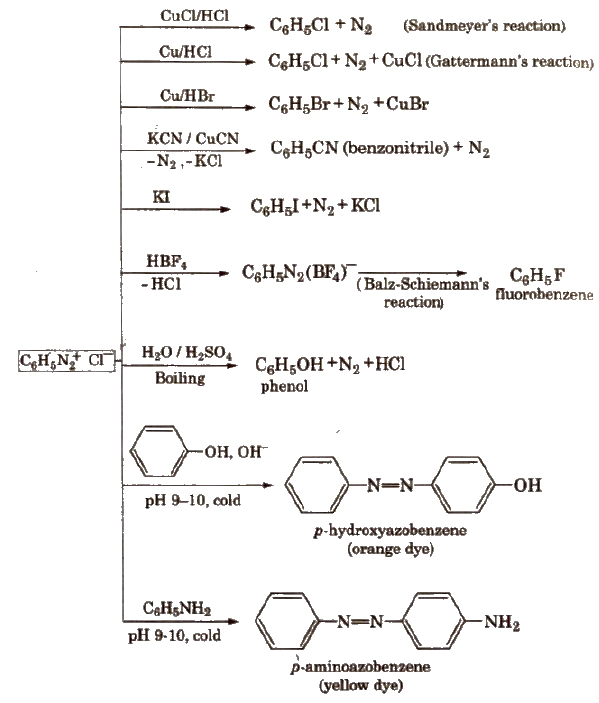
Benzene Diazonium Chloride (C6H5N2+;Cl–)
Preparation (Diazotisation reaction)
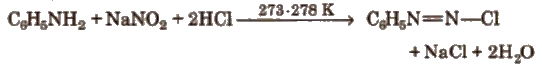
The excess acid in diazotisation reaction is necessary to maintain proper acidic medium for the reaction and to prevent combination of diazonium salt formed with the undiazotised amine.
Diazonium salts are prepared and used in aqueous solutions because in solid state, they explode.
Properties
It is a colourless crystalline solid, soluble in water. It has tendency to explode when dry.
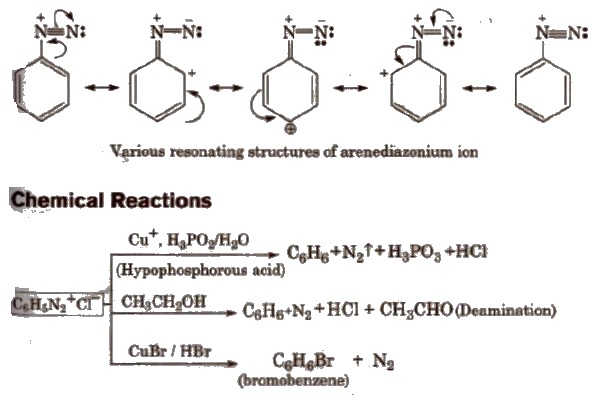
Stability of Arenediazonium salts
It is relatively more stable than the alkyldiazonium salt. The arenediazonium ion is resonance stabilised as is indicated by the following resonating structures:
Alkyl Cyanides’
These compound have formula RCN. These are the derivatives of RCN.
According to IUPAC system, cyanides are named as ‘alkane nitrile’, e.g.,
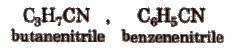
Methods of Preparation
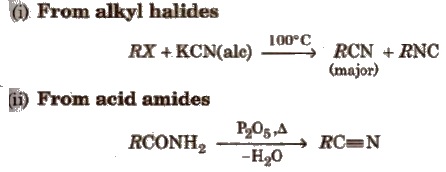
Physical properties
- These are neutral compound with pleasent odour, similar to bitter almonds.
- These are soluble in water as well as organic solvents.
- These are poisonous but less than HCN.
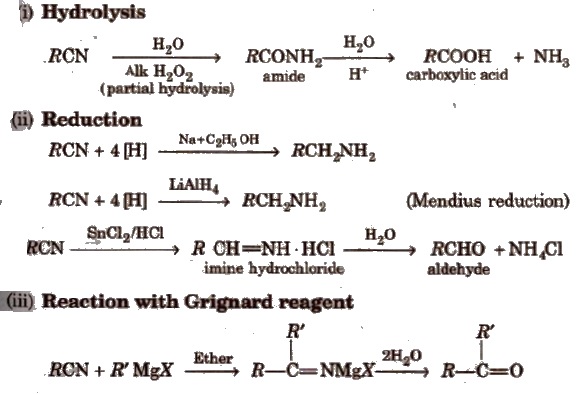
Chemical Properties
Alkyl iscoyanides (RNC)
Accordinlg to IUPAC system, these are named as ‘alkane isonitrile’
e.g., CH3NC methyl isonitrile
C6H5NC benzene isonitrile
Methods of Preparation
(a) From alkyl halides
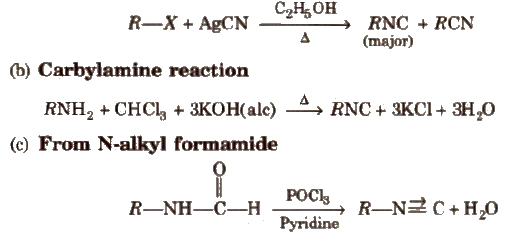
Physical Properties
- These are colourless unpleasent smelling liquids.
- These are soluble in organic solvents but insoluble in water.
Chemical Properties
(i) Hydrolysis
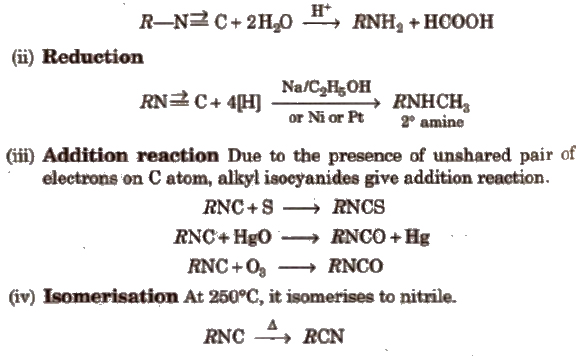
Nitro Compounds
These are obtained by replacing one H of hydrocarbon by -NO2 group.
These are named according to IUPAC system as ‘nitro alkane’.
Methods of Preparation
(i) From alkyl halides
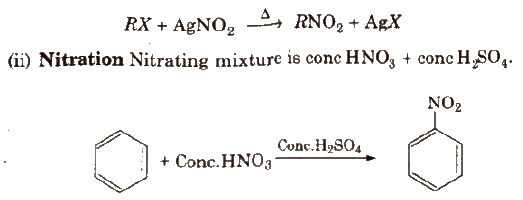
Physical Properties
- These are colourless pleasent smelling liquids.
- Their boiling point are much higher than isomeric alkyl nitriles.
- These are less soluble in water but readily soluble in organic solvents.
Chemical Properties
(i) Reduction With Sn/HCl or catalytic hydrogenation, nitroalkanes are reduced to amines.
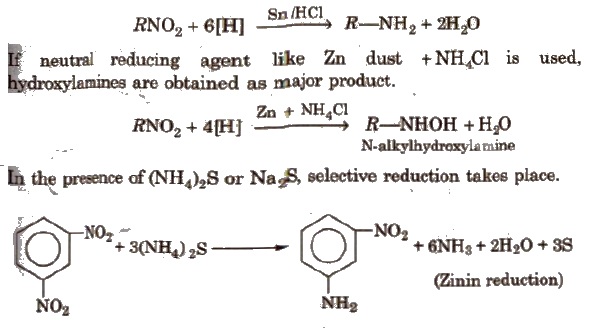
Nitrobenzene gives different prociucts with different reagents and in different mediums.
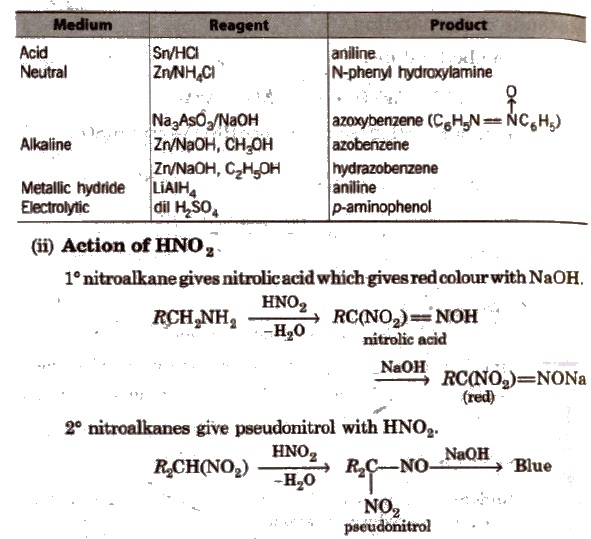
3° nitroalkanes does not react with HNO2
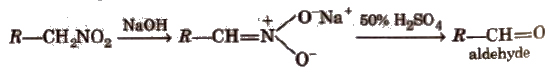
(iii) Nef carbonyl synthesis Na or K salt of 1° or 2° nitroalkanes give carbonyl compounds on acidification with 50% H2SO4 at room temperature. This reaction is called Nef carbonyl synthesis.
(iv) Electrophifie substitution On nitration, nitrobenzene gives m-dinitrobenzene (as -NO2 is a m-directing group and strongly deactivating).
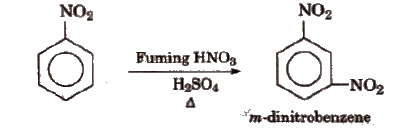
It does not give Friedel-Craft’s alkylation.
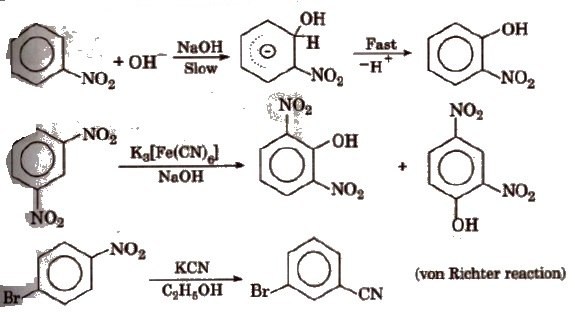
(v) Nucleophilic substitution reaction -NO2 group activates the ring towards nucleophilic substitution.
Class 12 Key Points, Important Questions & Practice Papers
Hope these notes helped you in your schools exam preparation. Candidates can also check out the Key Points, Important Questions & Practice Papers for various Subjects for Class 12 in both Hindi and English language form the link below.
Class 12 NCERT Solutions
Candidates who are studying in Class 12 can also check Class 12 NCERT Solutions from here. This will help the candidates to know the solutions for all subjects covered in Class 12th. Candidates can click on the subject wise link to get the same. Class 12 Chapter-wise, detailed solutions to the questions of the NCERT textbooks are provided with the objective of helping students compare their answers with the sample answers.
Class 12 Mock Test / Practice
Mock test are the practice test or you can say the blue print of the main exam. Before appearing in the main examination, candidates must try mock test as it helps the students learn from their mistakes. With the help of Class 12 Mock Test / Practice, candidates can also get an idea about the pattern and marking scheme of that examination. For the sake of the candidates we are providing Class 12 Mock Test / Practice links below.
Class 12 Exemplar Questions
Exemplar Questions Class 12 is a very important resource for students preparing for the Examination. Here we have provided Exemplar Problems Solutions along with NCERT Exemplar Problems Class 12. Question from very important topics is covered by Exemplar Questions for Class 12.
Class 12 Chemistry Maths Notes Physics Notes Biology Notes
To get study material, exam alerts and news, join our Whatsapp Channel.


