Class 12 Chemistry Chemistry in Everyday Life – Get here the Notes for Class 12 Chemistry in Everyday Life. Candidates who are ambitious to qualify the Class 12 with good score can check this article for Notes. This is possible only when you have the best CBSE Class 12 Chemistry Notes, study material, and a smart preparation plan. CBSE 2019 Class 12th Exam is approaching and candidates will have to make the best use of the time available towards the last stage of your CBSE Class 12th Chemistry Preparation. To help you with that, below we have provided the Notes of 12 Chemistry for topic Chemistry in Everyday Life.
- Class: 12th
- Subject: Chemistry
- Topic: Chemistry in Everyday Life
- Resource: Notes
CBSE Notes Class 12 Chemistry Chemistry in Everyday Life
Candidates who are pursuing in Class 12 are advised to revise the notes from this post. With the help of Notes, candidates can plan their Strategy for particular weaker section of the subject and study hard. So, go ahead and check the Important Notes for Class 12 Chemistry Chemistry in Everyday Life.
Medicines or Drugs
Chemicals which may be used for the treatment of diseases and for reducing the suffering from pain are called medicines or drugs.
The branch of science which makes use of chemicals for the treatment of disseases [therapeutic effect] is called chemotherapy.
Some important classes of drugs are
Antacid
The chemical substances which neutralize the excess acid in gastric juice and raise the pH to an appropriate level in stomach are raUed antacids.
The most commonly used antacids are weak bases such as sodium bicarbonate [sodium hydrogencarbonate, NaHCO3), magnesium hydroxide [Mg(OH)2] and aluminium hydroxide [Al(OH)3].
Generally liquid antacids are more effective than tablets because .. have more surface area available for interaction and neutralisation acid.
Milk is a weak antacid.
Histamine stimulates the secretion of pepsin and hydrochloric acid. The drug cimetidine [Tegamet] was designed to prevent the interaction of histamine with the receptors present in the stomach Cimetidine binds to the receptors tbat triggers the release of acid the stomach. This results in release of lesser amount of acid. Now ranitidine (zantac), omeprazole and lansoprazole are used hyperacidity.
Tranquilizers (Psychotherapeutic Drugs)
Chemical substances used for the treatment of stress, anxiety, irritability and mild or even severe mental diseases, are known as tranquilizers. These affect the central nervous system and induce sleep for the patients as well as eliminate the symptoms of emotional distress. They are the common constituents of sleeping pills.
Noradrenaline is one of the neurotransmitter that plays a role in mood change. If the level of noradrenaline is low, the signal sending activity becomes low, and the person suffers from depression. In such situations antidepressant drugs are required. These drugs inhibit the enzymes which catalyse the degradation of noradrenaline. If the enzyme is inhibited, this important neurotransmitter is slowly metabolized and can activate its receptor for longer periods of time, thus counteracting the effect of depression. Iproniazid and phenelzine are two such drugs.
Barbituric acid and its derivatives viz. veronal, amytal, nembutal, luminal, seconal are known as barbiturates. Barbiturates are hypnotic, i.e., sleep producing agents.
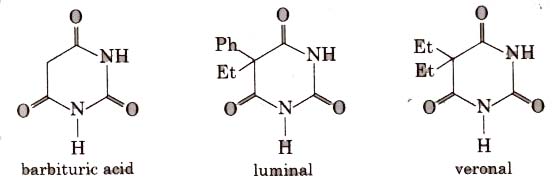
Equanil is used to control depression and hypertension.
Non-hypnotic chlorodiazepoxide and meprobamate are relatively mild tranquilizers suitable for relieving tension.
Analgesics
Medicines used for getting relief from pain are called analgesics. These are of two types :
1. Narcotics
Drugs which produce sleep and unconsciousness are called narcotics. Mhese are habit forming drugs. For example, morphine and codeine. Morphine diacetate is commonly known as heroin.
2. Non-narcotics
These are non-habit forming chemicals which reduce mild to moderate llatn such as headache, toothache, muscle and joint pain, etc. These are also termed as non-addicti,ve. These drugs do not produce sleep unconsciousness. Aspirin (2-acetoxybenzoic acid) is most commonly used analgesic with antipyretic properties. Now these days because its anti-blood clotting action, aspirin is widely used to
heart-attacks.
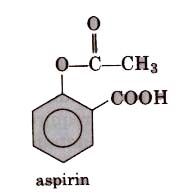
Aspirin is toxic for liver and sometimes also causes bleeding from- stomach. So, naproxen, ibuprofen, paracetamol,dichlorofenac sodium are other widely used analgesics.
Antipyretics
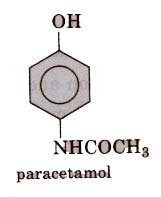
These are the chemical substance which reduce body temperature during high fever. Paracetamol, aspirin, phenacetin (4-hydroxy acetanilide), analgin and novalgin, etc., are common antipyretics. Out of these, paracetamol (4-acetamidophenol) is most common.
Antimicrobials
An antimicrobial tends to kill or prevent development of microbes CII inhibit the pathogenic action of microbes such as bacteria, fungi and virus selectively.
[Sulpha drugs constitute a group of drugs which are derivatives of sulphanilamide and have great antimicrobial capacity, thus, these are widely used against diseases such as dyptheria, dysentry, tubercu losis, etc.]
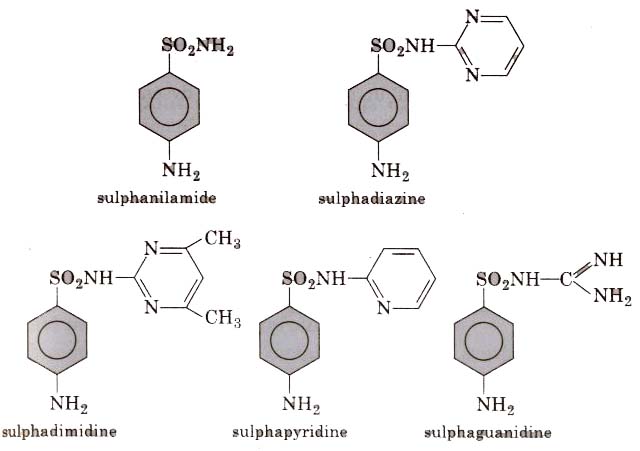
In structure these drugs are analogues of p-amino benzoic acid Different types of antimicrobial drugs are as follows :
1. Antibiotics
These are the substances (produced wholly or partially by chemical synthesis) which in low concentrations inhibit the growth of microorganisms or destroy them by intervening in their metabolic processes.
Antibiotics are products of microbial growth and thus, antibiotic therapy has been likened to ‘setting on.e thief against another’.
Antibiotics are of two types :
- Bactericidal antibiotics have cidal (killing) effect on microbes. For example, penicillin, ofloxacin, amino glycosides, etc.
- Bacteriostatic antibiotics have a static (inhibitory) effect on microbes. For example, erythromycin, tetracycline, chloramphenicol, etc
Penicillin was the first antibiotic discovered (by Alexander Fleming) in 1929. It is a narrow-spectrum antibiotic. Ampicillin and amoxicillin are semi-synthetic Illodifications of penicillin. Penicillin is not suitable to all persons and some Personsare allergic to it. Consequently, it is essential to test the patients for sensitivity (or allergy) to penicillin, before it is administered.
In India, penicillin is manufactured at Pimpri and Rishikesh (Uttarakhand).
Broad-spectrum antibiotics also called antibiotics, are antibiotics which are effective against different types of harmful microorganisms. e.g., Tetracycline, chloramphenicoltgiven in case of typhoid, dysentery, fever ofloxacin, etc.
2. Antiseptics
These are the chemicals which either kill or prevent the growth microorganisms. Antiseptics are applied to the living tissues such wounds, cuts, ulcers and skin diseases in the form of antiseptic creams like furacin and soframycin. e.g., Some important examples of antiseptics are
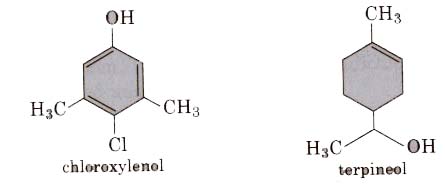
(i) Dettol is a mixture of chloroxylenol and terpineol.
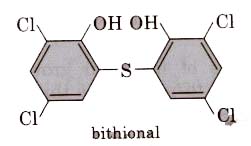
(ii) Bithional is added to soaps to impart antiseptic properties to reduce the odours produced by bacterial organic matter on the skin.
(iii) Tincture of iodine is a 2-3% solution of iodine in alcohol, which is a powerful antiseptic for wounds.
(iv) Iodoform (CHI3) is also used as an antiseptic for wounds.
(v) Boric acid in dilute aqueous solution is weak anIUS4!pa eyes.
3. Disinfectants
These are the chemical substances which kill microorganisms not safe to be applied to the living tissues. They are generally kill the microorganisms present on inanimate objects such as drainage systems, instruments, etc.
Some common examples of disinfectants are as follows :
(i) 1% phenol solution is disinfectant while in lower concentration 0.2% solution of phenol is antiseptic.
(ii) 0.2-0.4 ppm aqueous solution of chlorine is used for sterilisation of water to make it fit for drinking purpose.
(iii) SO2 at very low concentrations behaves like disinfectant.
(iv) Formaldehyde (HCHO) in the disinfecting rooms and operation gaseous theatres forms is used in hospitals. for
Antifertility Drugs
These are the chemical substances used to control the pregnancy. These are also called oral contraceptives. They belong to the class of natural products, known as steroids.
Birth control pills essentially contain a mixture of synthetic estrogen and progesterone derivatives. Norethindrone is widely used as antifertility drug.
Chemicals in Food
1. Artificial Sweetening Agents
Sucrose (table sugar) and fructose are the most widely used natural sweeteners. But they add to our calorie intake and promote tooth decay. To avoid these problems many people take artificial sweeteners.
Organic substances which have been synthesized in lab are known to be many times sweeter than cane sugar. Such compounds are known as artificial sweetening agents or artificial sweeteners.
Some important artificial sweeteners are given below :
(i) Saccharin (o-sulphobenzimide)
Discoveredby Johns- Hopkins in 1879 (University of USA).
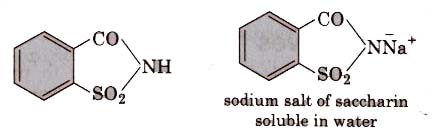
It is the most popular artificial sweetener. It is 550 times as sweet as cane Sugar, since it is insoluble in water, so it is sold in the market as its soluble or calcium salt.
It is non-biodegradable so excreted from the body in urine (unchanged). Its use is of great value for diabetic persons and people who need to control intake of calories.
(ii) Aspartame
It is the methyl ester of the dipeptide derived from phenylalanine aspartic acid. It is also known as ‘Nutra sweet’.
It decomposes at baking or cooking temperatures and hence, can used only in cold food and soft drinks.
Aspartame has the same amount of calories as sugar (4 cal per gram).
Aspartame should not be used by people suffering from the genetic disease known as PKU (phenyl ketone urea). Because in such people decomposition of aspartame gives phenylpyruvic acid. Accumulation ~ phenylpyruvic acid is harmful especially to infants due to brain damage and mental retardation.
(iii) Alitame
It is quite similar to aspartame but more stable than aspartame. It is 2000 times as sweet as sucrose. The main problem for such sweetenerf
is the control of sweeteness of the substance to which it is added because it is high potency sweetener.
(iv) Sucralose
It is a trichloro derivative of sucrose. It’s appearance and taste are like sugar. It is stable at cooking temperature. It is almost 600 times 88 sweet as sucrose. However, it neither provides calories nor causes toot.1i decay.
(v) Cyclamate
It is N-cyclohexylsulphamate. It is only 20 times sweeter than cane sugar.

2. Food Preservatives
These are the chemical substances added to food to prevent their spoilage due to microbial growth (bacteria, yeasts and moulds) and to retain their nutritive value for longer periods .
The most commonly used preservatives include table salt, supgar, vegetable oil, vinegar, citric acid. spices and sodium benzonate (C6H5COONA). Salts of sorbic acid and propanoic acid are also used” preservatives for cheese, baked food, pickles, meat and fish products.
1. Sodium benzoate is metabolised by conversion into hioppuric acid (C6H5CONHCH2COOH), which is ultimately urine. It is used in soft drinks and acidic foods.
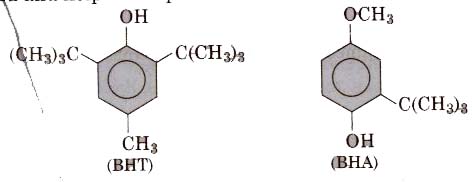
2. Antioxidants like BHT(butylated hydroxytoluene) and BRA butylated hydroxyanisole) retard the action of oxygen on the food and help in the preservation of food materials.
Cleansing Agents
The word detergent means cleansing agent. Actually detergent word is derived from Latin word ‘detergere’ means “to wipe off”, Cleansing agents are the substance which remove dirt and have cleansing action in water. These are also called surfactants.
Detergents can be classified into two types.
- Soapy detergents or soaps, and
- Non-soapy detergents or soapless soap.
1. Soaps
Soaps are sodium or potassium salts of higher fatty acids (containing 15-18 carbon atoms) e.g., stearic acid, oleic acid and palmitic acid. Sodium salts of fatty acids are known as hard soaps while the potassium salts of fatty acids are known as soft soaps.
Hard soaps are prepared by cheaper oil and NaOH while soft soaps are Jlrepared by oil of good quality and KOH. The soft soaps do not contain freealkali, produce more lather and are used as toilet soaps, shaving SOapsand shampoos.
Preparation of soaps
Soaps containing ‘Sodium salts are formed by heating fat (glyceryl ester ~fatty acid) with aqueous sodium hydroxide solution. This reaction is known as saponification.
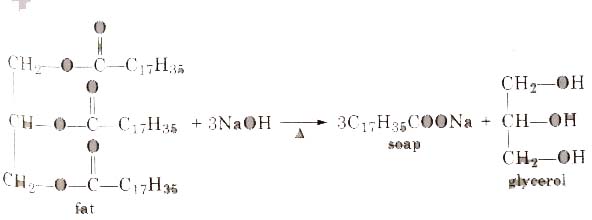
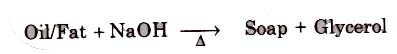
The solution left after removing the soap contains glycerol, which can be recovered by fractional distillation. To improve the quality of soaps desired colours, perfumes and medicinal chemical substances, added.
Types of Soaps
Different kind of soaps are made by using different raw materials.
- Toilet soaps These are prepared by using better grade of fat or oil and care is taken to remove excess alkali. Colour and perfumes are added to make these more attractive.
- Floating soaps These can be prepared by beating tiny bubbles into the product before it hardens.
- Transparent soaps These are made by dissolving the in ethanol and then evaporating the excess solvent.
- Medicated soaps Medicated soaps are prepared by some antiseptics like dettol or bithional.
- Shaving soaps These contain glycerol to prevent drying. A gum called rosin is added while making them. It forms sodium rosinate which lather well.
- Laundry soaps These sodium silicate, borax and contain sodium fillers like carbonate. sodium rosins
- Soap Chips These are made by running a thin sheet of melted soap on a cool cylinder and scraping off the soaps in small broken pieces.
- Soap grannules These are dried miniature soap bubbles.
- Soap powder and scouring soaps These contain a scouring agent (abrasive) such as powdered pumice or finely divided sand and builders like sodium carbonate and trisodium phosphate. Builders make the soaps act more quickly.
Disadvantages of Soaps
Soap is good cleansing agent and is 100% biodegradable microorganisms present in sewage water can completely oxidise soap to CO2, As a result, it does not create any pollution problem. However soaps have two disadvantages:
(i) Soaps cannot be used in hard water since calcium magnesium ions present in hard water produce curdy precipitates of calcium and magnesium soaps.
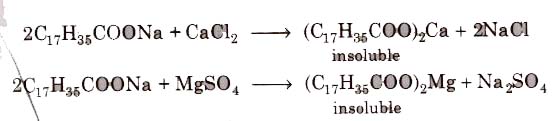
These insoluble soaps separate as scum in water and causes hinderance to washing because the precipitate adheres onto the fibre of the cloth as gummy mass. Thus, a lot of soap is wasted if water. is hard.
(ii) Soaps cannot be used in acidic solutions since acids precipitate the insoluble free fatty acids which adhere to the fabrics and thus, reduce the ability of soaps to remove oil and grease from fabrics.

Soapless Soap/Synthetic Detergents
Synthetic detergents have all the properties of soaps but actually does not contain any soap, so they are known as ‘soapless soaps’.
Straight chain alkyl group containing detergents are biodegradable whereas branched chain alkyl group containing detergents are non-biodegradable.
Unlike soaps, synthetic detergents can be used in both soft and hard water. This is due to the reason that calcium and magnesium salts of detergents like their sodium salts are also soluble in water. Synthetic
detergents are mainly classified into three categories:
1. Anionic Detergents
These are sodium salts of sulphonated long chain alcohols or hydrocarbons.
(i) Alkyl hydrogen sulphates formed by treating long chain alcohols with concentrated sulphuric acids are neutralised with alkali to form anionic detergents.
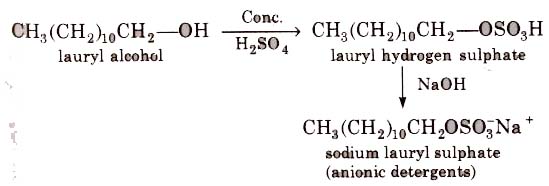
(ii) Alkyl benzene sulphonates are obtained by neutralising alkyl benzene sulphonic acids with alkali.
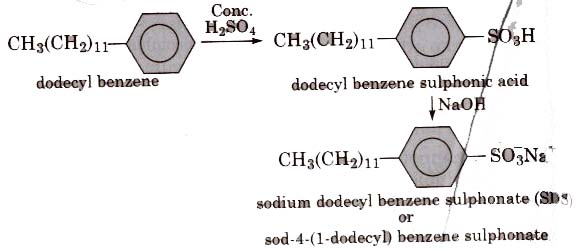
In such detergents, the anionic part of the molecule is involved in the cleansing action.
They are mostly used for household work and in tooth paste
2. Cationic Detergents
These are quaternary ammonium salts of arnines with acetates, chlorides or bromides as anion. For example,
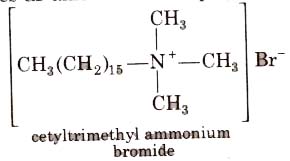
Cationic detergents are used in hair conditioner. They have germicidal properties but are expensive therefore, these are of limited use.
3. Non-ionic Detergents
Such detergents does not contain any ion in their constitution. One such detergent can be obtained by reaction of stearic acid and polyethylene glycol.
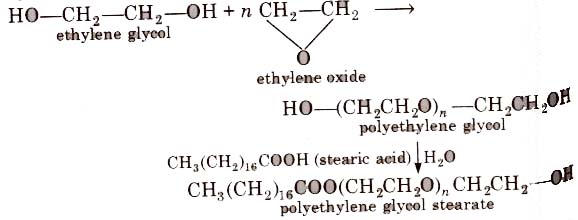
Liquid dish washing detergents are non-ionic type, Mechanism of cleansing action of this type of detergents is the same as that of soaps.
Advantages of syntbeti.c detergents over soaps
1. Synthetic detergents can be used even in case of hard water whereas soaps fail to do so.
2. Synthetic detergents can be used in the acidic medium while soaps cannot because of their hydrolysis to free acids.
3. Synthetic detergents are more soluble in water and hence, form better lather than soaps.
4. Synthetic detergents have a stronger cleansing action than soaps.
Chemistry in Colouring Matter
The natural or synthetic colouring matter which are used in solution to stain materials especially fabrics are called dyes.
All colouring substances are not dyes, e.g., azobenzene, a coloured substance does ‘not act as dye.
A dye have following characteristics :.
- It must have a suitable colour.
- It can be fixed on the fabric either directly or with the help of mordant.
- It must be resistant to the action of water, acid and alkalies. The groups, responsible for colour, are called chromophore, e.g.,
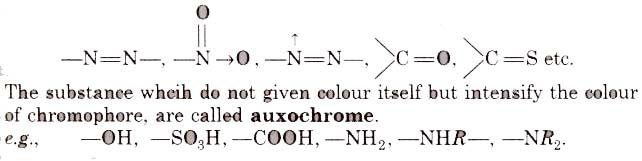
Classification of Dyes on the Basis of Constitution
(i) Nitro or nitroso dye Chromophore NO2 or NO group, Auxochrome = -OH group, e.g., picric acid, martius yellow, Gambine, naphthol yellow-S.
(ii) Azo dye, e.g., bismark brown, methyl orange, methyl red, congo red, etc.
(iii) Anthraquinone dye e.g., alizarin
(iv) Indigo is the oldest known dye. Other examples are tyrian purple, indigosol.
(v) Phthalein dye e.g., phenolphthalein, fluorescein, eosin, mercurochrome.
(vi) Triarybnethane dye, e.g., malachite green, rosaniline.
Classification of Dyes on the Basis of Application
(i) Direct dyes These dyes applied directly to fibre and are more useful to the fabrics containing H-bonding like cotton, rayon, wool, silk and nylon, e.g., martius yellow, congo reu/etc.
(ii) Acid dyes These are water soluble and contain porar/acidic groups which interact with the basic group of e.g., Orange-I, congo red, methyl orange, etc. These dyes does not have affinity for cotton but are used for silk, wool, etc.
(iii) Basic dyes These dyes contain basic group (like NHz group) and react with anionic sites present on the fabric. These are used to dye nylons and polyester, e.g., butter yellow, magenta (rosaniline), aniline yellow, etc.
(iv) Vat dyes Being water insoluble, these cannot be applied directly. These are first reduced to a colourless soluble form by a reducing agent in large vats and then, applied to fabrics. After applying, these are oxidised to insoluble coloured form by exposure. to air or some oxidising agents, e.g., Indigo, tyrian purple, etc.
(v) Mordant dyes These are applied with the help of a binding material (e.g., metal ion, tannic acid or metal hydroxide) called mordant. Depending upon the metal ion used, the same dye can give different colours. Alizarin is an important example of such dyes.
(vi) Ingrain dye These dyes are synthesised directly on the fabric. These are water insoluble and particularly suitable for cotton fibres. Azo dyes belong to this group of dye.
Chemistry in Cosmetics
Cosmetics are used for decorating, beautifying or improving complexion of skin. Some of the cosmetics of daily use are as
1. Creams
These are stable emulsions of oils or fats in water and contain emmollients (to prevent water loss) and humectants (to attract water) as two fundamental components.
2. Perfumes
These solutions have pleasent odour and invariably consist of three ingredients: a vehicle (ethanol + H20), fixative e.g., sandalwood oil, benzoin, glyceryl diacetate etc.) and odour producing substance (e.g., terpenoids like linalool, anisaldehyde (p-methoxy- benzaldehyde etc.)
3. Talcum Powder
It is used to reduce irritation of skin. Talc (Mg3(OH)2Si4O10), chalk, Zno, zinc sterate and a suitable perfume are the constituents of talcum powder.
4. Deodorants
These are applied to mask the body odour. These possess antibacterial properties. Aluminium salts, ZnO, Zn02, (C17H35COO)2Zn can be used in deodorant preparation.
Rocket Propellants
Substances used for launching rockets are called rocket propellants. These are the combination of an oxidiser and a fuel.
Depending upon the physical states of oxidiser and fuels, rocket propellants are classified as
1. Solid Propellants
These are further divided into two classes
(i) Composite propellants In these propellants, fuel is polymeric binder such as polyurethane or polybutadiene and oxidiser is ammonium per chlorate or potassium perchlorate.
Class 12 Key Points, Important Questions & Practice Papers
Hope these notes helped you in your schools exam preparation. Candidates can also check out the Key Points, Important Questions & Practice Papers for various Subjects for Class 12 in both Hindi and English language form the link below.
Class 12 NCERT Solutions
Candidates who are studying in Class 12 can also check Class 12 NCERT Solutions from here. This will help the candidates to know the solutions for all subjects covered in Class 12th. Candidates can click on the subject wise link to get the same. Class 12 Chapter-wise, detailed solutions to the questions of the NCERT textbooks are provided with the objective of helping students compare their answers with the sample answers.
Class 12 Mock Test / Practice
Mock test are the practice test or you can say the blue print of the main exam. Before appearing in the main examination, candidates must try mock test as it helps the students learn from their mistakes. With the help of Class 12 Mock Test / Practice, candidates can also get an idea about the pattern and marking scheme of that examination. For the sake of the candidates we are providing Class 12 Mock Test / Practice links below.
Class 12 Exemplar Questions
Exemplar Questions Class 12 is a very important resource for students preparing for the Examination. Here we have provided Exemplar Problems Solutions along with NCERT Exemplar Problems Class 12. Question from very important topics is covered by Exemplar Questions for Class 12.
Class 12 Chemistry Maths Notes Physics Notes Biology Notes
To get study material, exam alerts and news, join our Whatsapp Channel.




