Class 11 Physics Kinetic Theory of Gases – Get here the Notes for Class 11 Physics Kinetic Theory of Gases. Candidates who are ambitious to qualify the Class 11 with good score can check this article for Notes. This is possible only when you have the best CBSE Class 11 Physics study material and a smart preparation plan. To assist you with that, we are here with notes. Hope these notes will helps you understand the important topics and remember the key points for exam point of view. Below we provided the Notes of Class 11 Physics for topic Kinetic Theory of Gases.
- Class: 11th
- Subject: Physics
- Topic: Kinetic Theory of Gases
- Resource: Notes
CBSE Notes Class 11 Physics Kinetic Theory of Gases
Candidates who are pursuing in Class 11 are advised to revise the notes from this post. With the help of Notes, candidates can plan their Strategy for particular weaker section of the subject and study hard. So, go ahead and check the Important Notes for Class 11 Physics Kinetic Theory of Gases from this article.
Assumptions of Kinetic Theory of Gases
- Every gas consists of extremely small particles known as molecules. The molecules of a given gas are all identical but are different from those of another gas.
- The molecules of a gas are identical spherical, rigid and perfectly elastic point masses.
- Their molecular size is negligible in comparison to intermolecular distance (10-9 m).
- The speed of gas molecules lies between zero and infinity (very high speed).
- The distance covered by the molecules between two successive collisions is known as free path and mean of all free path is known as mean free path.
- The number of collision per unit volume in a gas remains constant.
- No attractive or repulsive force acts between gas molecules.
- Gravitational to extremely attraction among the molecules is ineffective due small masses and very high speed of molecules.
Gas laws
Assuming permanent gases to be ideal, through experiments, it was established that gases irrespective of their nature obey the following laws.
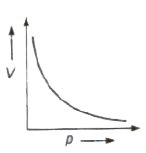
Boyle’s Law
At constant temperature the volume (V) of given mass of a gas is inversely proportional to its pressure (p), i.e.,
V ∝ 1/p ⇒ pV = constant
For a given geas, p1V1 = p2V2
Charles’ Law
At constant pressure the volume (V) of a given mass of gas is directly proportional to its absolute temperature (T), i.e.,
V ∝ T ⇒ V / T = constant
For a given gas, V1/T1 = V2/T2
At constant pressure the volume (V) of a given mass of a gas increases or decreases by 1/273.15 of its volume at 0°C for each 1°C rise or fall in temperature.
Volume of the gas at t°Ce
Vt = V0 (1 + t/273.15)
where V0 is the volume of gas at 0°C.
Gay Lussacs’ or Regnault’s Law
At constant volume the pressure p of a given mass of gas is directly proportional to its absolute temperature T, i.e. ,
p ∝ T ⇒ V/T = constant
For a given gas,
p1/T1 = p2/T2
At constant volume (V) the pressure p of a given mass of a gas increases or decreases by 1/273.15 of its pressure at 0°C for each l°C rise or fall in temperature.
Volume of the gas at t°C, pt = p0 (1 + t/273.15)
where P0 is the pressure of gas at 0°C.
Avogadro’s Law
Avogadro stated that equal volume of all the gases under similar conditions of temperature and pressure contain equal number molecules. This statement is called Avogadro’s hypothesis. According Avogadro’s law
(i) Avogadro’s number The number of molecules present in 1g mole of a gas is defined as Avogadro’s number.
NA = 6.023 X 1023 per gram mole
(ii) At STP or NTP (T = 273 K and p = 1 atm 22.4 L of each gas has 6.023 x 1023 molecules.
(iii) One mole of any gas at STP occupies 22.4 L of volume.
Standard or Perfect Gas Equation
Gases which obey all gas laws in all conditions of pressure and temperature are called perfect gases.
Equation of perfect gas pV=nRT
where p = pressure, V = volume, T = absolute temperature, R = universal gas constant and n = number of moles of a gas.
Universal gas constant R = 8.31 J mol-1K-1.
Real Gases
Real gases deviate slightly from ideal gas laws because
- Real gas molecules attract one another.
- Real gas molecules occupy a finite volume.
Real or Van der Waal’s Gas Equation
(p + a/V2) (V – b) = RT
where a and b are called van der Waals’ constants.
Pressure due to an ideal gas is given by
p = (1/3).(mn/V). c2 = 1/3 ρ c2
For one mole of an ideal gas
P = (1/3).(M/V).c2
where, m = mass of one molecule, n = number of molecules, V = volume of gas, c = (c12 + c22 + … + cn2) / n allde root mean square (rrns) velocity of the gas molecules and M = molecular weight of the gas. If p is the pressure of the gas and E is the kinetic energy per unit volume is E, then
p = (2/3).E
Kinetic Energy of a Gas
(i) Average kinetic energy of translation per molecule of a gas is given by
E = (3/2) kt
where k = Boltzmann’s constant.
(ii) Average kinetic energy of translation per mole of a gas is given by
E = (3/2) Rt
where R = universal gas constant.
(iii) For a given gas kinetic energy
E ∝ T ⇒ E1/E2 = T1/T2
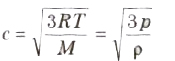
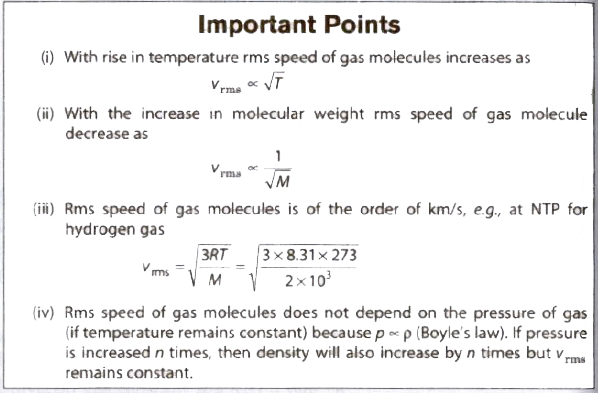
(iv) Root mean square (rms) velocity of the gas molecules is given by
(v) For a given gas c ∝ √T
(vi) For different gases c ∝1/√M
(vii) Boltzmann’s constant k = R/N
where R is ideal gas constant and N = Avogadro number.
Value of Boltzmann’s constant is 1.38 x 10-28 J/K.
(viii) The average speed of molecules of a gas is given by
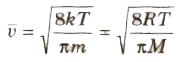
(ix) The most probable speed of molecules of a gas is given by
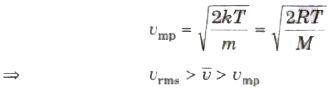
Degree of Freedom
The degree of freedom for a dynamic system is the number of directions in which it can move freely or the number of coordinates required to describe completely the position and configuration of the system.
It is denoted by for N.
Degree of freedom of a system is given by
f or N = 3A – R
where A = number of particles in the system and R = number of independent relations.
Degree of Freedom
- For monoatomic gas = 3
- For diatomic gas = 5
- For non-linear triatomic gas = 6
- For linear triatomic gas = 7
Specific heat of a gas
(a) At constant volume, CV = f/2 R
(b) At constant pressure, cp = (f/2 + 1)R
(c) Ratio of specific heats of a gas at constant pressure and at constant volume is given by
γ = 1 + 2/f
Mean Free Path
The average distance travelled by a molecule between two successive collisions is called mean free path (γ).
Mean free path is given by
γ = kT / √2 π σ2p
where σ = diameter of the molecule, p = pressure of the gas,
T = temperature and k = Botlzmann’s constant.
Mean free path
λ ∝ T and λ ∝ 1/p
Brownian Motion
The continuous random motion of the particles of microscopic size suspended in air or any liquid, is called Brownian of microscopic motion.
Brownian suspended motion in both is observed with many liquids and gases.
Brownian motion is due to the unequal bombardment of the suspended Particles by the molecules of the surrounding medium.
Class 11 Key Points, Important Questions & Practice Papers
Hope these notes helped you in your schools exam preparation. Candidates can also check out the Key Points, Important Questions & Practice Papers for various Subjects for Class 11 in both Hindi and English language form the link below.
Class 11 NCERT Solutions
Candidates who are studying in Class 11 can also check Class 11 NCERT Solutions from here. This will help the candidates to know the solutions for all subjects covered in Class 11th. Candidates can click on the subject wise link to get the same. Class 11 Chapter-wise, detailed solutions to the questions of the NCERT textbooks are provided with the objective of helping students compare their answers with the sample answers.
Class 11 Mock Test / Practice
Mock test are the practice test or you can say the blue print of the main exam. Before appearing in the main examination, candidates must try mock test as it helps the students learn from their mistakes. With the help of Class 11 Mock Test / Practice, candidates can also get an idea about the pattern and marking scheme of that examination. For the sake of the candidates we are providing Class 11 Mock Test / Practice links below.
Class 11 Exemplar Questions
Exemplar Questions Class 11 is a very important resource for students preparing for the Examination. Here we have provided Exemplar Problems Solutions along with NCERT Exemplar Problems Class 11. Question from very important topics is covered by Exemplar Questions for Class 11.
CBSE Notes for Class 11 Physics Notes Biology Notes Maths Notes Chemistry Notes
To get study material, exam alerts and news, join our Whatsapp Channel.

