Class 12 Physics Nuclear Physics – Get here the Notes for Class 12 Physics Nuclear Physics. Candidates who are ambitious to qualify the Class 12 with good score can check this article for Notes. This is possible only when you have the best CBSE Class 12 Physics study material and a smart preparation plan. To assist you with that, we are here with notes. Hope these notes will helps you understand the important topics and remember the key points for exam point of view. Below we provided the Notes of Class 12 Physics for topic Nuclear Physics
- Class: 12th
- Subject: Physics
- Topic: Nuclear Physics
- Resource: Notes
CBSE Notes Class 12 Physics Nuclear Physics
Candidates who are pursuing in Class 12 are advised to revise the notes from this post. With the help of Notes, candidates can plan their Strategy for particular weaker section of the subject and study hard. So, go ahead and check the Important Notes for Class 12 Physics Nuclear Physics
Nucleus
The entire positive charge and nearly the entire mass of atom is concentrated in a very small space called the nucleus of an atom.
The nucleus consists of protons and neutrons. They are called nucleons.
Terms Related to Nucleus
(i) Atomic Number The number of protons in the nucleus of an atom of the element is called atomic number (Z) of the element.
(ii) Mass Number The total number of protons and neutrons present inside the nucleus of an atom of the element is called mass number (A) of the element.
(iii) Nuclear Size The radius of the nucleus R ∝ A1/3
⇒ R = Ro A1/3
where, Ro = 1.1 * 10-15 m is an empirical constant.
(iv) Nuclear Density Nuclear density is independent of mass number and therefore same for all nuclei.
ρ = mass of nucleus / volume of nucleus ⇒ ρ = 3m / 4π R3o
where, m = average mass of a nucleon.
(v) Atomic Mass Unit It is defined as 1 / 12th the mass of carbon nucleus.
It is abbreviated as arnu and often denoted by u. Thus
1 amu = 1.992678 * 10-26 / 12 kg
= 1.6 * 10-27 kg = 931 Me V
Isotopes
The atoms of an element having same atomic number but different mass numbers. are called isotopes.
e.g., 1H1, 1H2, 1H3 are isotopes of hydrogen.
Isobars
The atoms of different elements having same mass numbers but different atomic numbers, are called isobars.
e.g., 1H3, 2He3 and 10Na22, 10Ne22 are isobars.
Isotones
The atoms of different elements having different atomic numbers and different mass numbers but having same number of neutrons, are called isotones.
e.g., 1H3, 2He4 and 6C14, 8O16 are isobars.
Isomers
Atoms having the same mass number and the same atomic number but different radioactive properties are called isomers,
Nuclear Force
The force acting inside the nucleus or acting between nucleons is called nuclear force.
Nuclear forces are the strongest forces in nature.
- It is a very short range attractive force.
- It is non-central. non-conservative force.
- It is neither gravitational nor electrostatic force.
- It is independent of charge.
- It is 100 times that of electrostatic force and 1038 times that of gravitational force.
According to the Yukawa, the nuclear force acts between the nucleon due to continuous exchange of meson particles.
Mass Defect
The difference between the sum of masses of all nucleons (M) mass of the nucleus (m) is called mass defect.
Mass Defect (Δm) = M – m = [Zmp + (A – Z)mn – mn]
Nuclear Binding Energy
The minimum energy required to separate the nucleons up to an infinite distance from the nucleus, is called nuclear binding energy.
Nuclear binding energy per nucleon = Nuclear binding energy / Total number of nucleons
Binding energy, Eb = [Zmp + (A – Z) mn – mN]c2
Packing Fraction (P)
p = (Exact nuclear mass) – (Mass number) / Mass number
= M – A / M
The larger the value of packing friction. greater is the stability of the nucleus.
[The nuclei containing even number of protons and even number of neutrons are most stable.
The nuclei containing odd number of protons and odd number of neutrons are most instable.]
Radioactivity
The phenomena of disintegration of heavy elements into comparatively lighter elements by the emission of radiations is called radioactivity. This phenomena was discovered by Henry Becquerel in 1896.
Radiations Emitted by a Radioactive Element
Three types of radiations emitted by radioactive elements
(i) α-rays
(ii) β-rays
(iii) γ – rays
α-rays consists of α-particles, which are doubly ionised helium ion.
β-rays are consist of fast moving electrons.
γ – rays are electromagnetic rays.
[When an α – particle is emitted by a nucleus its atomic number decreases by 2 and mass number decreases by 4.

When a β -particle is emitted by a nucleus its atomic number is Increases by one and mass number remains unchanged.

When a γ – particle is emitted by a nucleus its atomic number and mass number remain unchanged
Radioactive Decay law
The rate of disintegration of radioactive atoms at any instant is directly proportional to the number of radioactive atoms present in the sample at that instant.
Rate of disintegration ( – dN / dt) ∝ N
– dN / dt = λ N
where λ is the decay constant.
The number of atoms present undecayed in the sample at any instant N = No e-λt
where, No is number of atoms at time t = 0 and N is number of atoms at time t.
Half-life of a Radioactive Element
The time is which the half number of atoms present initially in any sample decays, is called half-life (T) of that radioactive element.
Relation between half-life and disintegration constant is given by
T = log2e / λ = 0.6931 / λ
Average Life or Mean Life(τ)
Average life or mean life (τ) of a radioactive element is the ratio of total life time of all the atoms and total number of atoms present initially in the sample.
Relation between average life and decay constant τ = 1 / λ
Relation between half-life and average life τ = 1.44 T
The number of atoms left undecayed after n half-lifes is given by
N = No (1 / 2)n = No (1 / 2) t/T
where, n = t / T, here t = total time.
Activity of a Radioactive Element
The activity of a radioactive element is equal to its rate of disintegration.
Activity R = ( – dN / dt)
Activity of the sample after time t,
R = Ro e -λt
Its SI unit is Becquerel (Bq).
Its other units are Curie and Rutherford.
1 Curie = 3.7 * 1010 decay/s
1 Rutherford = 106 decay/s
Nuclear Fission
The process of the splitting of a heavy nucleus into two or more lighter nuclei is called nuclear fission.
When a slow moving neutron strikes with a uranium nucleus (92U235), it splits into 56Ba141 and 36Kr92 along with three neutrons and a lot of energy.
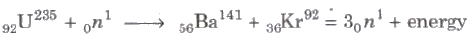
Nuclear Chain Reaction
If the particle starting the nuclear fission reaction is produced as a product and further take part in the nuclear fission reaction, then a chain of fission reaction started, which is called nuclear chain reaction.
Nuclear chain reaction are of two types
(i) Controlled chain reaction
(ii) Uncontrolled chain reaction
Nuclear Reactor
The main parts of a nuclear reactor are following
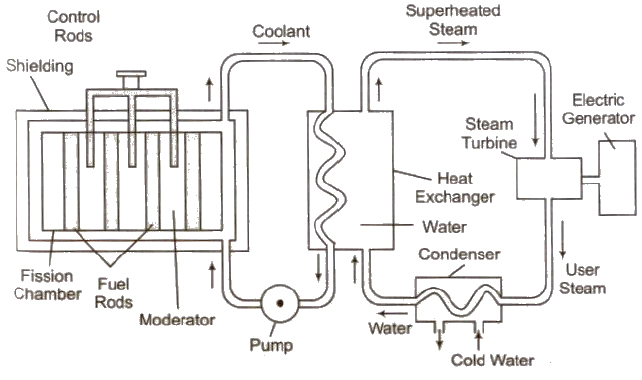
(i) Fuel Fissionable materials like 92U235, 92U238, 94U239 are used as fuel.
(ii) Moderator Heavy water, graphite and beryllium oxide are used to slower down fast moving neutrons.
(iii) Coolant The cold water, liquid oxygen, etc. are used to remove heat generated in the fission process.
(iv) Control rods Cadmium or boron rods are good absorber of neutrons and therefore used to control the fission reaction.
Atom bomb working is based on uncontrolled chain reaction.
Nuclear Fusion
The process of combining of two lighter nuclei to form one heavy nucleus, is called nuclear fusion.
Three deuteron nuclei (1H2) fuse, 21.6 MeV is energy released and nucleus of helium (2He4) is formed.
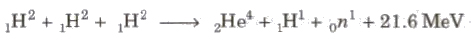
In this process, a large amount of energy is released.
Nuclear fusion takes place at very high temperature approximately about 107 K and at very high pressure 106 atmosphere.
Hydrogen bomb is based on nuclear fusion.
The source of Sun’s energy is the nuclear fusion taking place at sun.
Thermonuclear Energy
The energy released during nuclear fusion is know as thermonuclear energy. Protons are needed for fusion while neutrons are needed for fission process.
Class 12 Key Points, Important Questions & Practice Papers
Hope these notes helped you in your schools exam preparation. Candidates can also check out the Key Points, Important Questions & Practice Papers for various Subjects for Class 12 in both Hindi and English language form the link below.
Class 12 NCERT Solutions
Candidates who are studying in Class 12 can also check Class 12 NCERT Solutions from here. This will help the candidates to know the solutions for all subjects covered in Class 12th. Candidates can click on the subject wise link to get the same. Class 12 Chapter-wise, detailed solutions to the questions of the NCERT textbooks are provided with the objective of helping students compare their answers with the sample answers.
Class 12 Mock Test / Practice
Mock test are the practice test or you can say the blue print of the main exam. Before appearing in the main examination, candidates must try mock test as it helps the students learn from their mistakes. With the help of Class 12 Mock Test / Practice, candidates can also get an idea about the pattern and marking scheme of that examination. For the sake of the candidates we are providing Class 12 Mock Test / Practice links below.
Class 12 Exemplar Questions
Exemplar Questions Class 12 is a very important resource for students preparing for the Examination. Here we have provided Exemplar Problems Solutions along with NCERT Exemplar Problems Class 12. Question from very important topics is covered by Exemplar Questions for Class 12.
To get study material, exam alerts and news, join our Whatsapp Channel.


