Candidates can download NCERT Exemplar Class 9 Science Unit 4 from this page. The exemplar has been provided by the National Council of Educational Research & Training (NCERT) and the candidates can check it from below for free of cost. It contains objective, very short answer type, short answer type, and long answer type questions. Along with it, the answer for each question has also been provided. From the NCERT Exemplar Class 9 Science Unit 4, candidates can understand the level and type of questions that are asked in the exam.
NCERT Exemplar Class 9 Science Unit 4 Structure Of The Atom
NCERT Class 9 Science Unit 4 is for Structure Of The Atom. The type of questions that will be asked from NCERT Class 9 Science Unit 4 are displayed in the below provided NCERT Exemplar Class 9 Science Unit 4. With the help of it, candidates can prepare well for the examination.
Also Check NCERT Solutions for Class 9 Science
Multiple Choice Questions
- Which of the following correctly represent the electronic distribution in the Mg atom?
(a) 3, 8, 1
(b) 2, 8, 2
(c) 1, 8, 3
(d) 8, 2, 2 - Rutherford’s ‘alpha (α) particles scattering experiment’ resulted in to discovery of
(a) Electron
(b) Proton
(c) Nucleus in the atom
(d) Atomic mass - The number of electrons in an element X is 15 and the number of neutrons is 16. Which of the following is the correct representation of the element?
(a) 3115X
(b) 3116X
(c) 1615X
(d) 1516X - Dalton’s atomic theory successfully explained
(i) Law of conservation of mass
(ii) Law of constant composition
(iii) Law of radioactivity
(iv) Law of multiple proportion
(a) (i), (ii) and (iii)
(b) (i), (iii) and (iv)
(c) (ii), (iii) and (iv)
(d) (i), (ii) and (iv) - Which of the following statements about Rutherford’s model of atom are correct?
(i) considered the nucleus as positively charged
(ii) established that the α–particles are four times as heavy as a hydrogen atom
(iii) can be compared to solar system
(iv) was in agreement with Thomson’s model
(a) (i) and (iii)
(b) (ii) and (iii)
(c) (i) and (iv)
(d) only (i) - Which of the following are true for an element?
(i) Atomic number = number of protons + number of electrons
(ii) Mass number = number of protons + number of neutrons
(iii) Atomic mass = number of protons = number of neutrons
(iv) Atomic number = number of protons = number of electrons
(a) (i) and (ii)
(b) (i) and (iii)
(c) (ii) and (iii)
(d) (ii) and (iv) - In the Thomson’s model of atom, which of the following statments are correct?
(i) the mass of the atom is assumed to be uniformaly distributed over the atom
(ii) the positive charge is assumed to be uniformaly distributed over the atom
(iii) the electrons are uniformaly distributed in the positively charged sphere
(iv) the electrons attract each other to stabilise the atom
(a) (i), (ii) and (iii)
(b) (i) and (iii)
(c) (i) and (iv)
(d) (i), (iii) and (iv) - Rutherford’s α–particle scattering experiment showed that
(i) electrons have negative charge
(ii) the mass and positive charge of the atom is concentrated in the nucleus
(iii) neutron exists in the nucleus
(iv) most of the space in atom is empty
Which of the above statements are correct?
(a) (i) and (iii)
(b) (ii) and (iv)
(c) (i) and (iv)
(d) (iii) and (iv) - The ion of an element has 3 positive charges. Mass number of the atom is 27 and the number of neutrons is 14. What is the number of electrons in the ion?
(a) 13
(b) 10
(c) 14
(d) 16 - Identify the Mg2+ ion from the Fig.4.1 where, n and p represent the number of neutrons and protons respectively

- In a sample of ethyl ethanoate (CH3COOC2H5) the two oxygen atoms have the same number of electrons but different number of neutrons. Which of the following is the correct reason for it?
(a) One of the oxygen atoms has gained electrons
(b) One of the oxygen atoms has gained two neutrons
(c) The two oxygen atoms are isotopes
(d) The two oxygen atoms are isobars. - Elements with valency 1 are
(a) always metals
(b) always metalloids
(c) either metals or non-metals
(d) always non-metals - The first model of an atom was given by
(a) N. Bohr
(b) E. Goldstein
(c) Rutherford
(d) J.J. Thomson - An atom with 3 protons and 4 neutrons will have a valency of
(a) 3
(b) 7
(c) 1
(d) 4 - The electron distribution in an aluminium atom is
(a) 2, 8, 3
(b) 2, 8, 2
(c) 8, 2, 3
(d) 2, 3, 8 - Which of the following in Fig. 4.2 do not represent Bohr’s model of an atom correctly?

(a) (i) and (ii)
(b) (ii) and (iii)
(c) (ii) and (iv)
(d) (i) and (iv) - Which of the following statement is always correct?
(a) An atom has equal number of electrons and protons.
(b) An atom has equal number of electrons and neutrons.
(c) An atom has equal number of protons and neutrons.
(d) An atom has equal number of electrons, protons and neutrons. - Atomic models have been improved over the years. Arrange the following atomic models in the order of their chronological order
(i) Rutherford’s atomic model
(ii) Thomson’s atomic model
(iii) Bohr’s atomic model
(a) (i), (ii) and (iii)
(b) (ii), (iii) and (i)
(c) (ii), (i) and (iii)
(d) (iii), (ii) and (i)
Short Answer Type Questions
- Is it possible for the atom of an element to have one electron, one proton and no neutron. If so, name the element.
- Write any two observations which support the fact that atoms are divisible.
- Will 35Cl and 37Cl have different valencies? Justify your answer.
- Why did Rutherford select a gold foil in his α–ray scattering experiment?
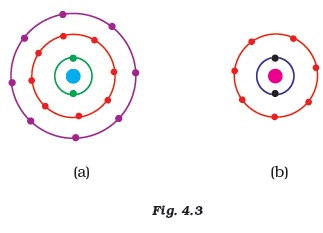
- Find out the valency of the atoms represented by the Fig. 4.3 (a) and (b).
- One electron is present in the outer most shell of the atom of an element X. What would be the nature and value of charge on the ion formed if this electron is removed from the outer most shell?
- Write down the electron distribution of chlorine atom. How many electrons are there in the L shell? (Atomic number of chlorine is 17).
- In the atom of an element X, 6 electrons are present in the outermost shell. If it acquires noble gas configuration by accepting requisite number of electrons, then what would be the charge on the ion so formed?
- What information do you get from the Fig. 4.4 about the atomic number, mass number and valency of atoms X, Y and Z? Give your answer in a tabular form.

- In response to a question, a student stated that in an atom, the number of protons is greater than the number of neutrons, which in turn is greater than the number of electrons. Do you agree with the statement? Justify your answer.
- Calculate the number of neutrons present in the nucleus of an element X which is represented as 3115X.
- Match the names of the Scientists given in column A with their contributions towards the understanding of the atomic structure as given in column B

- The atomic number of calcium and argon are 20 and 18 respectively, but the mass number of both these elements is 40. What is the name given to such a pair of elements?
- Complete the Table 4.1 on the basis of information available in the symbols given below
(a) 3535Cl
(b) 126C
(c) 8135Br}
- Helium atom has 2 electrons in its valence shell but its valency is not 2, Explain.
- Fill in the blanks in the following statements
(a) Rutherford’s α-particle scattering experiment led to the discovery of the ———
(b) Isotopes have same ———but different———.
(c) Neon and chlorine have atomic numbers 10 and 17 respectively. Their valencies will be———and———respectively.
(d) The electronic configuration of silicon is ———and that of sulphur is ———. - An element X has a mass number 4 and atomic number 2. Write the valency of this element?
Long Answer Type Questions
- Why do Helium, Neon and Argon have a zero valency?
- The ratio of the radii of hydrogen atom and its nucleus is ~ 105. Assuming the atom and the nucleus to be spherical, (i) what will be the ratio of their sizes? (ii) If atom is represented by planet earth ‘Re’ = 6.4 × 106m, estimate the size of the nucleus.
- Enlist the conclusions drawn by Rutherford from his α-ray scattering experiment.
- In what way is the Rutherford’s atomic model different from that of Thomson’s atomic model?
- What were the drawbacks of Rutherford’s model of an atom?
- What are the postulates of Bohr’s model of an atom?
- Show diagramatically the electron distributions in a sodium atom and a sodium ion and also give their atomic number.
- In the Gold foil experiment of Geiger and Marsden, that paved the way for Rutherford’s model of an atom, ~ 1.00% of the α-particles were found to deflect at angles > 50o. If one mole of α-particles were bombarded on the gold foil, compute the number of α-particles that would deflect at angles less than 50°.
| « Previous | Next » |
Answers
To get study material, exam alerts and news, join our Whatsapp Channel.









