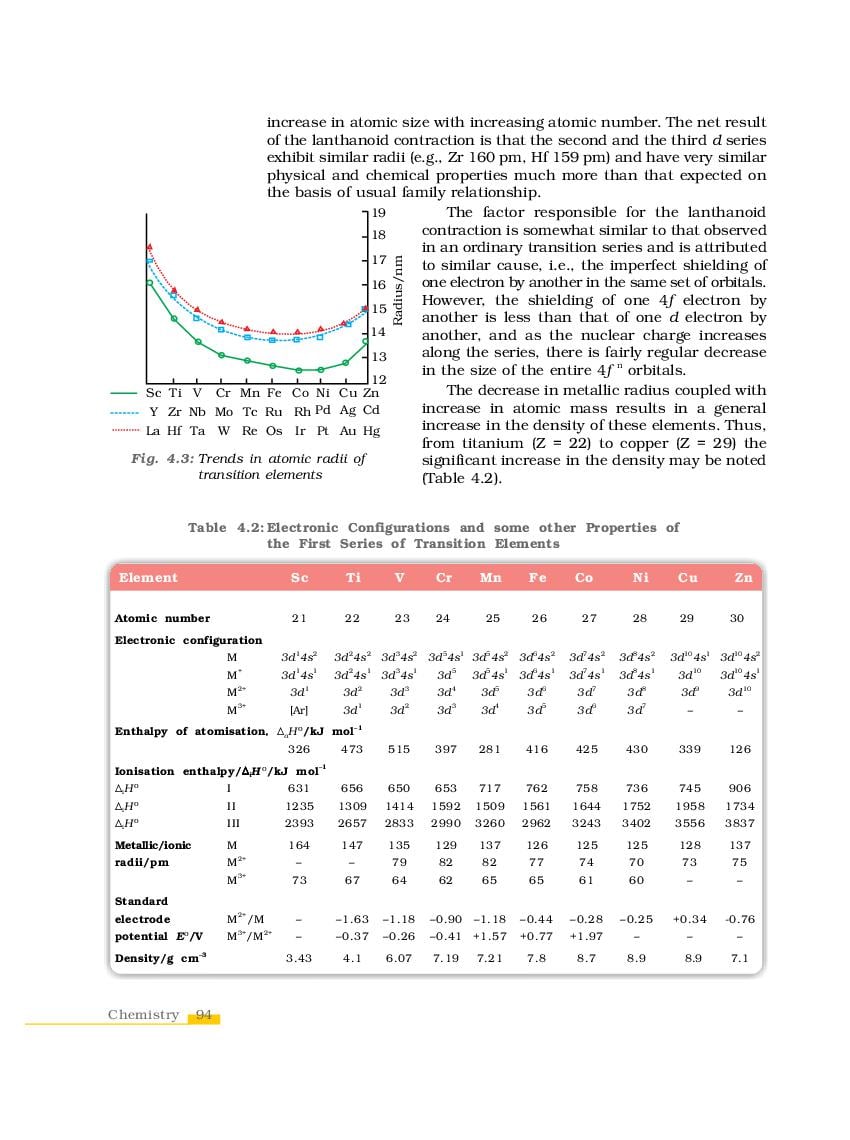
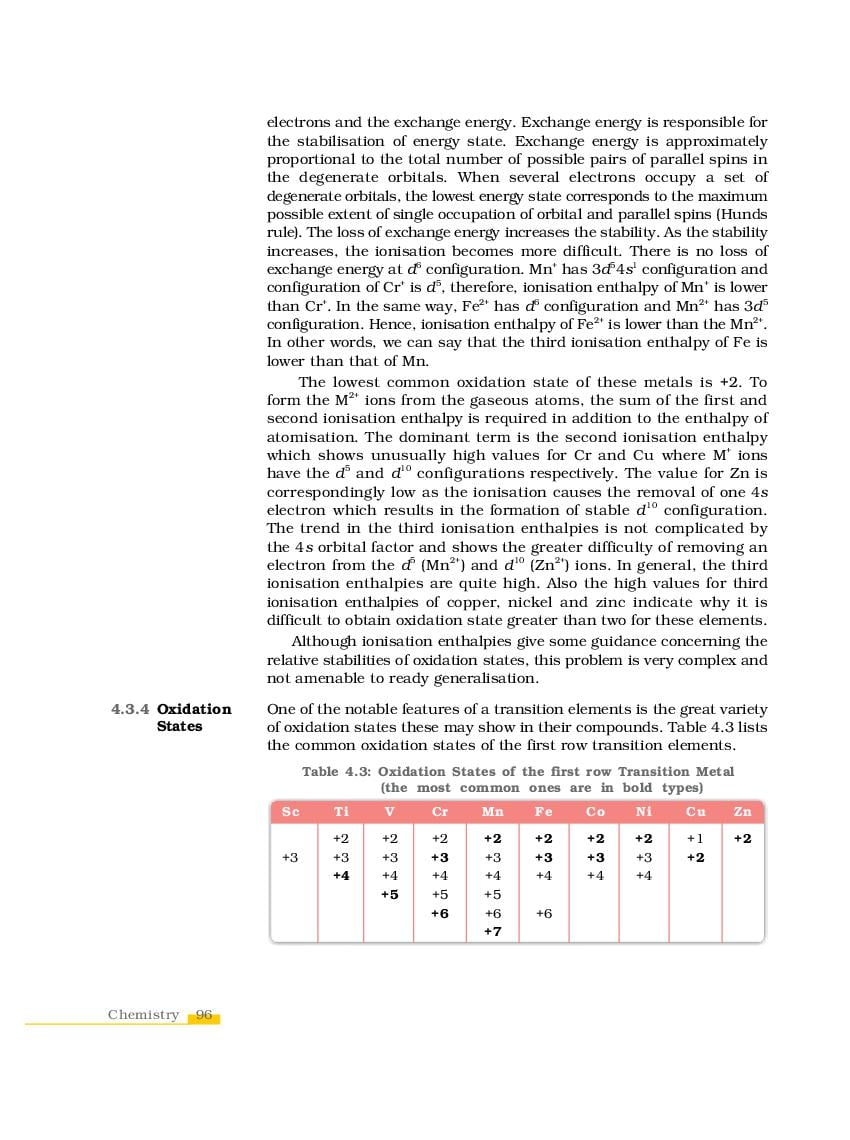
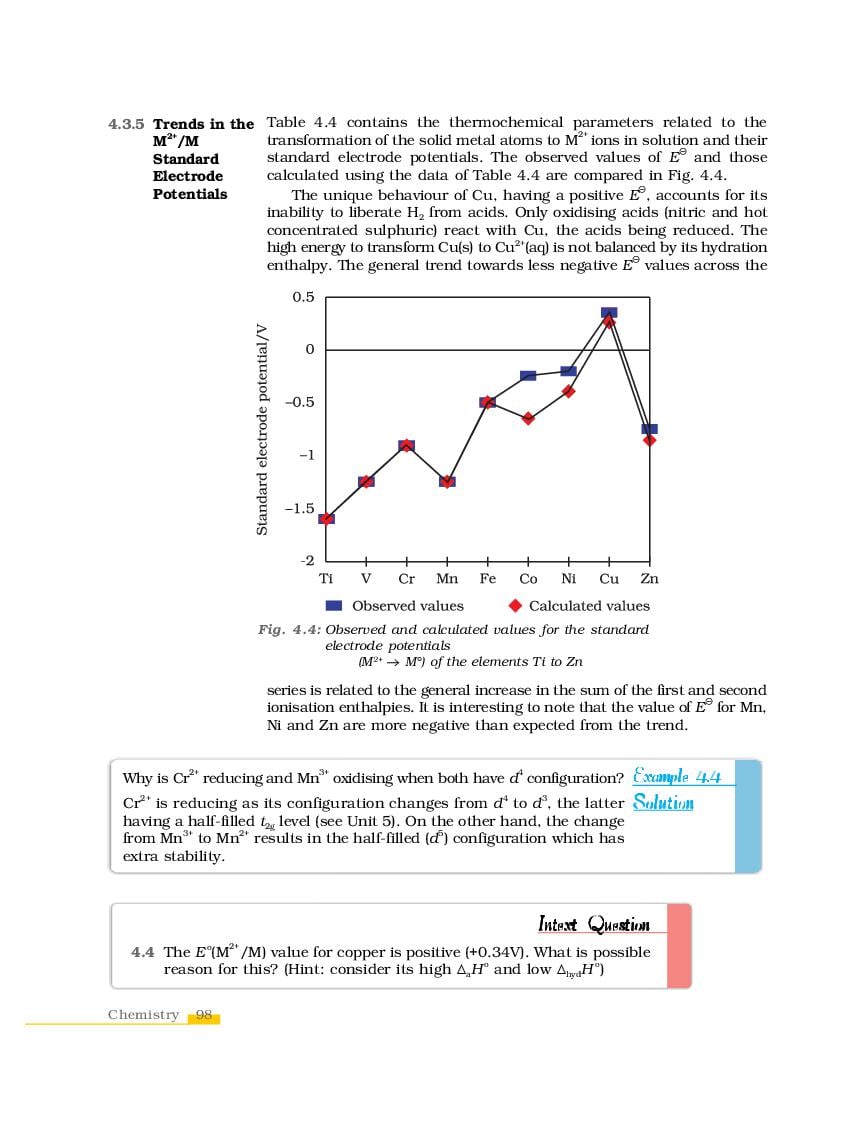
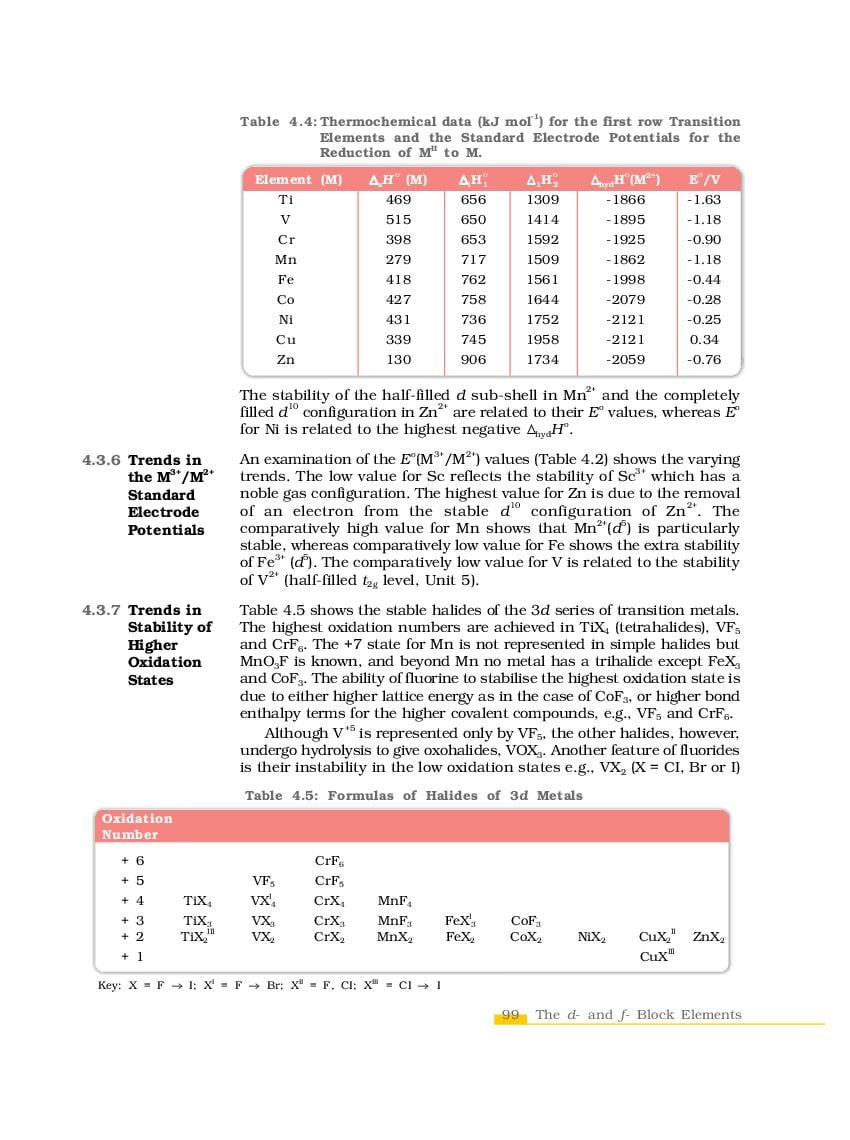
NCERT Book Class 12 Chemistry Chapter 4 The d-and f-Block Elements is here. You can read and download Class 12 Chemistry Chapter 4 PDF from this page of aglasem.com. The d-and f-Block Elements is one of the many lessons in NCERT Book Class 12 Chemistry in the new, updated version of 2023-24. So if you are in 12th standard, and studying Chemistry textbook (named Chemistry), then you can read Ch 4 here and afterwards use NCERT Solutions to solve questions answers of The d-and f-Block Elements.
NCERT Book Class 12 Chemistry Chapter 4 The d-and f-Block Elements
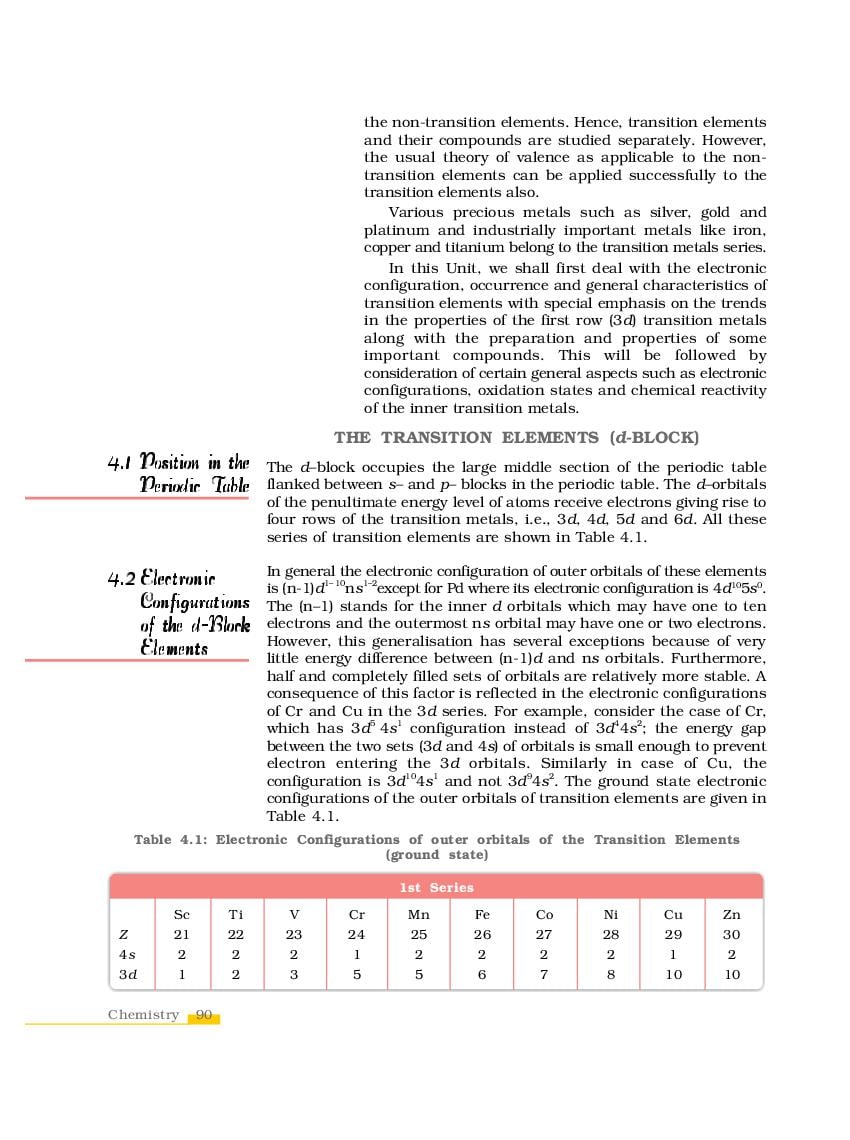
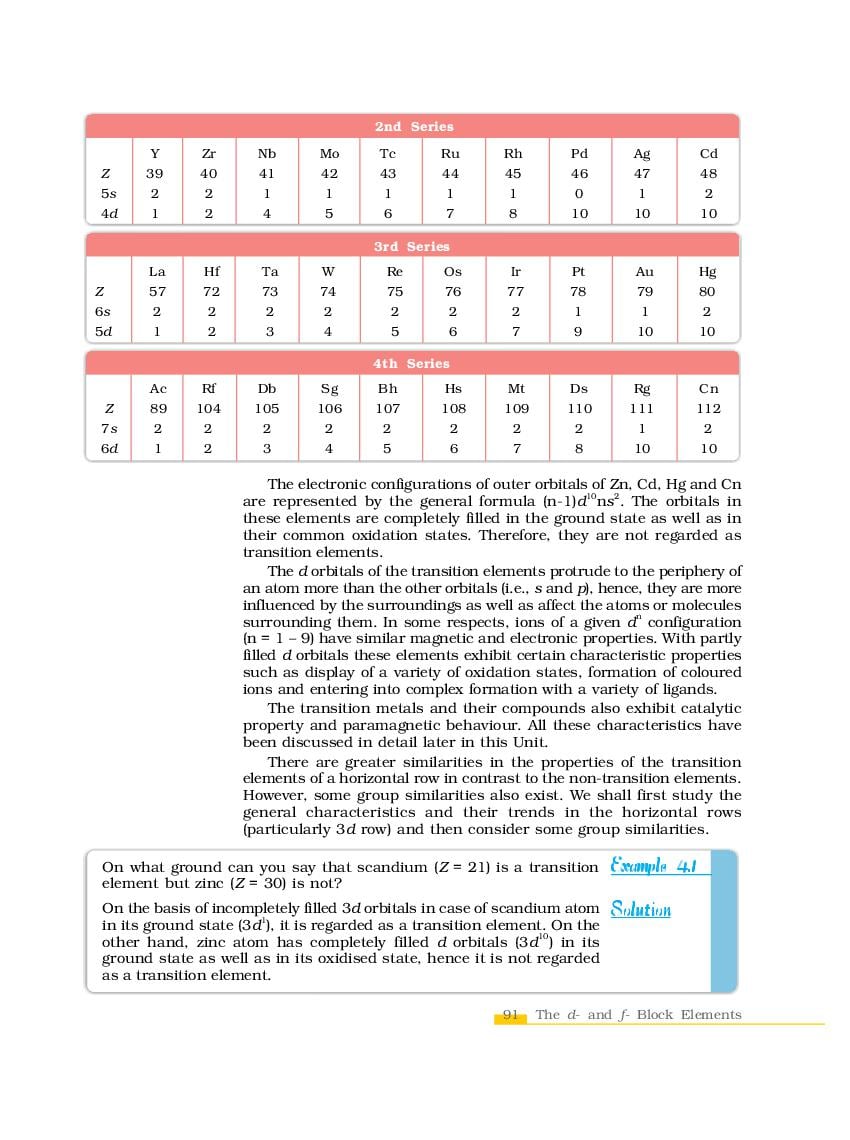
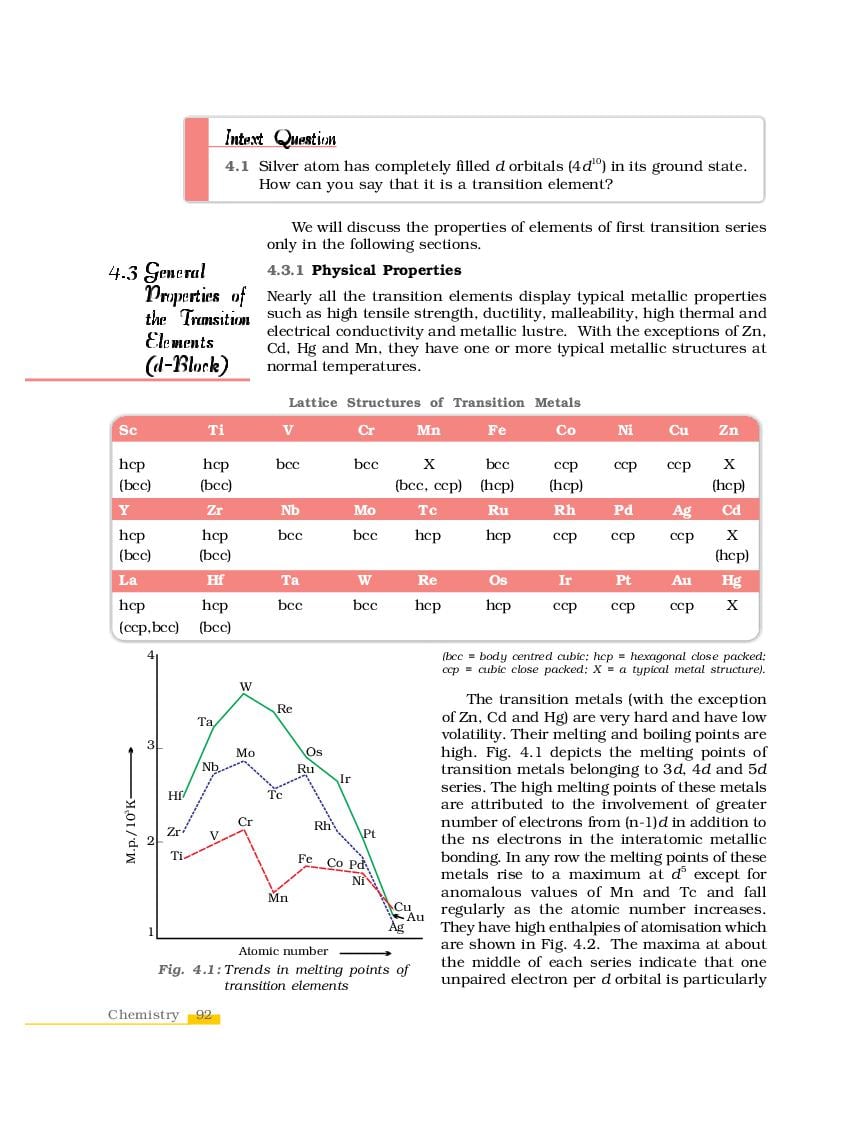
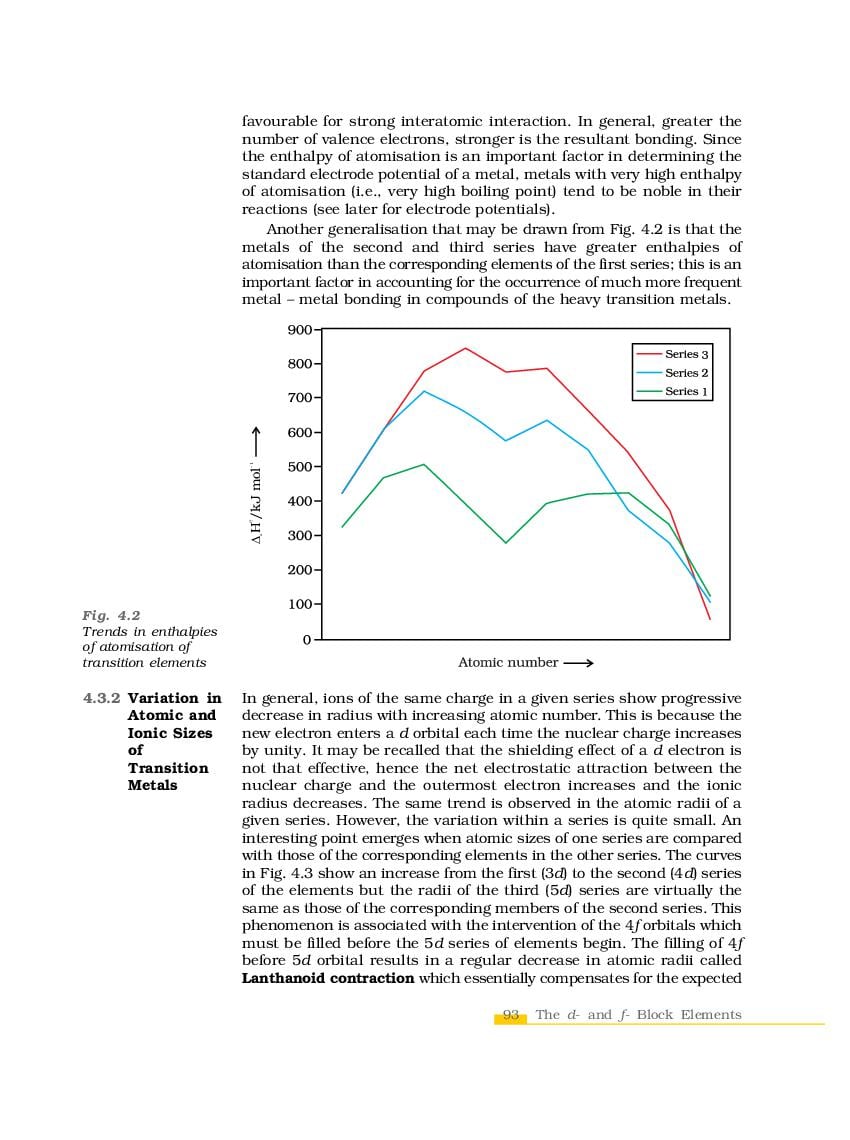
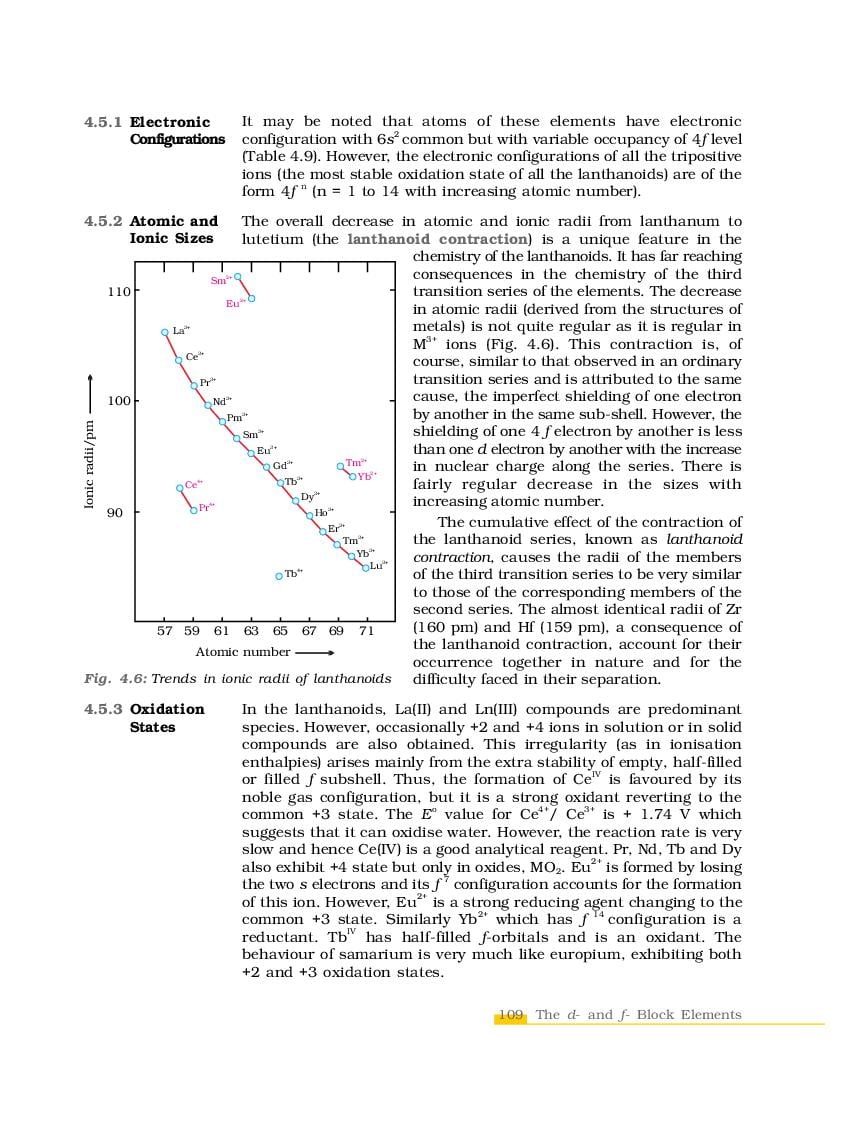
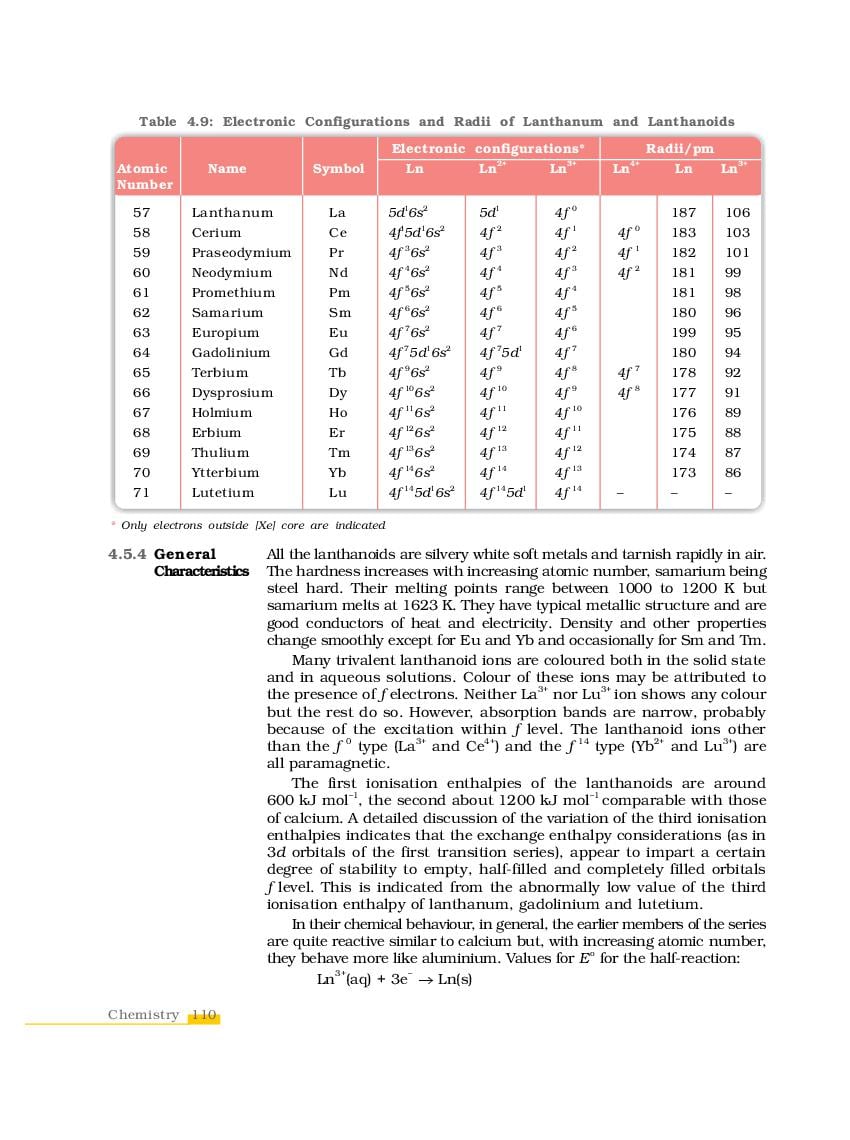
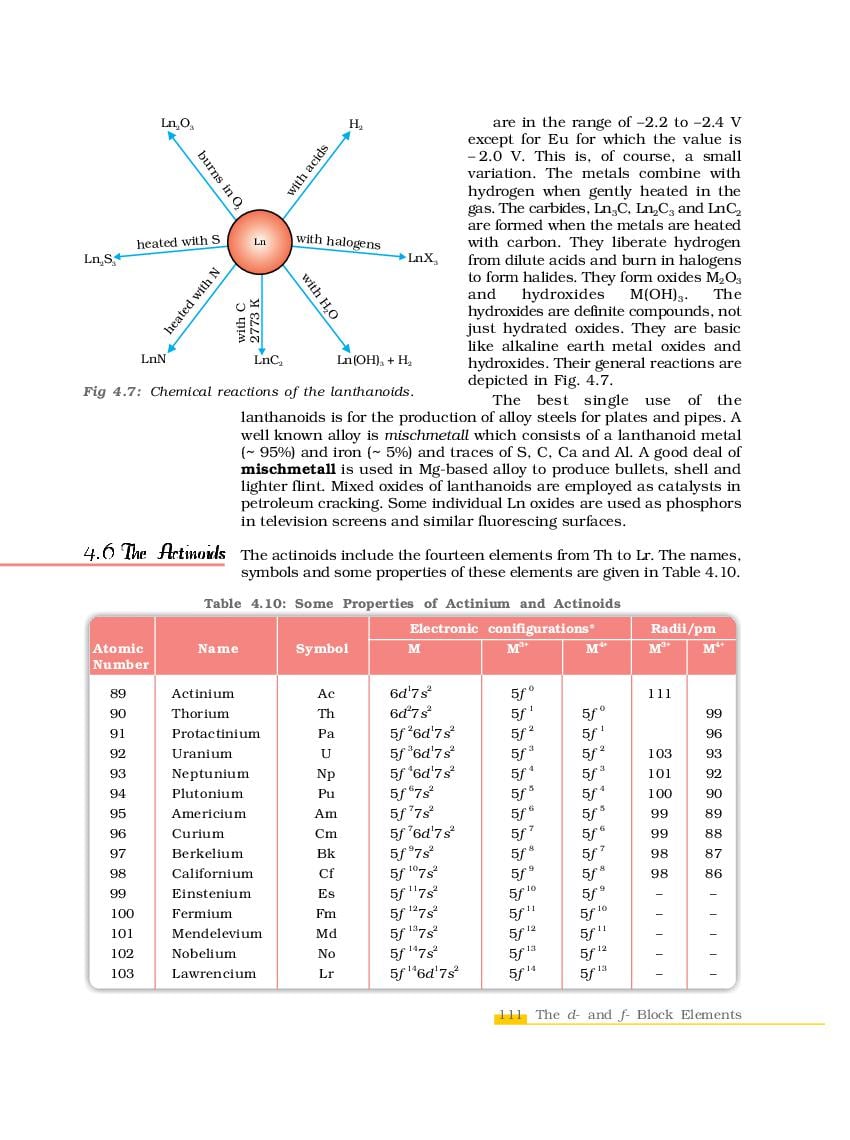
The complete Chapter 4, which is The d-and f-Block Elements, from NCERT Books for Class 12 Chemistry is as follows.
NCERT Book Class 12 Chemistry Chapter 4 The d-and f-Block Elements PDF Download Link – Click Here To Download The Complete Chapter PDF
NCERT Book Class 12 Chemistry Full Book PDF Download Link – Click Here To Download The Complete Book PDF
NCERT Book Class 12 Chemistry Chapter 4 The d-and f-Block Elements PDF
The direct link to download class 12 Chemistry NCERT Book PDF for chapter 4 The d-and f-Block Elements is given above. However if you want to read the complete lesson on The d-and f-Block Elements then that is also possible here at aglasem. So here is the complete class 12 Chemistry Ch 4 The d-and f-Block Elements.
NCERT Book Class 12 Chemistry Chapter 4 The d-and f-Block Elements View DownloadNCERT Book for Class 12 Chemistry
Besides the chapter on The d-and f-Block Elements, you can read or download the NCERT Class 12 Chemistry PDF full book from aglasem. Here is the complete book:
- Chapter 1 : The Solid State
- Chapter 2 : Solutions
- Chapter 3 : Electrochemistry
- Chapter 4 : Chemical Kinetics
- Chapter 5 : Surface Chemistry
- Chapter 6 : General Principles and Processes of Isolation of Elements
- Chapter 7 : The p-Block Elements
- Chapter 8 : The d-and f-Block Elements
- Chapter 9 : Coordination Compounds
- Chapter 10 : Haloalkanes and Haloarenes
- Chapter 11 : Alcohols, Phenols and Ethers
- Chapter 12 : Aldehydes, Ketones and Carboxylic Acids
- Chapter 13 : Amines
- Chapter 14 : Biomolecules
- Chapter 15 : Polymers
- Chapter 16 : Chemistry in Everyday Life
NCERT Books for Class 12
Similarly all the subject-wise class 12 books at aglasem.com are as follows.
- NCERT Book Class 12 Accountancy
- NCERT Book Class 12 Biology
- NCERT Book Class 12 Business Studies
- NCERT Book Class 12 Chemistry
- NCERT Book Class 12 Economics
- NCERT Book Class 12 English
- NCERT Book Class 12 Geography
- NCERT Book Class 12 Hindi
- NCERT Book Class 12 History
- NCERT Book Class 12 Maths
- NCERT Book Class 12 Physics
- NCERT Book Class 12 Political Science
- NCERT Book Class 12 Psychology
- NCERT Book Class 12 Sociology
NCERT Books
All class-wise books of National Council of Educational Research and Training are as follows.
- NCERT Books for Class 1
- NCERT Books for Class 2
- NCERT Books for Class 3
- NCERT Books for Class 4
- NCERT Books for Class 5
- NCERT Books for Class 6
- NCERT Books for Class 7
- NCERT Books for Class 8
- NCERT Books for Class 9
- NCERT Books for Class 10
- NCERT Books for Class 11
- NCERT Books for Class 12
Class 12 Chemistry Chapter 4 The d-and f-Block Elements NCERT Textbook – An Overview
The highlights of this The d-and f-Block Elements chapter PDF are as follows.
| Aspects | Details |
|---|---|
| Class | 12 |
| Subject | Chemistry |
| Book | Chemistry |
| Chapter Number | Ch 4 |
| Chapter Name | The d-and f-Block Elements |
| Book Portion Here | NCERT Book Class 12 Chemistry Ch 4 The d-and f-Block Elements |
| Download Format | |
| Version | NCERT Book (New, Updated) 2023-24 |
| Complete Book | NCERT Book Class 12 Chemistry |
| All Class 12 Books | NCERT Books for Class 12 |
| All Textbooks | NCERT Books |
| NCERT Books in Hindi | NCERT Books for Class 12 in Hindi |
| NCERT Solutions | NCERT Solutions for Class 12 |
| More Study Material | Class 12 |
If you have any queries on NCERT Book Class 12 Chemistry Chapter 4 The d-and f-Block Elements, then please ask in comments below. And if you found the Class 12 Chemistry Chapter 4 The d-and f-Block Elements PDF helpful, then do share with your friends on telegram, facebook, whatsapp, twitter, and other social media! :)
To get study material, exam alerts and news, join our Whatsapp Channel.