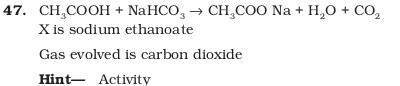Candidates can download NCERT Exemplar Class 10 Science Unit 4 from this page. The exemplar has been provided by the National Council of Educational Research & Training (NCERT) and the candidates can check it from below for free of cost. It contains objective, very short answer type, short answer type, and long answer type questions. Along with it, the answer for each question has also been provided. From the NCERT Exemplar Class 10 Science Unit 4, candidates can understand the level and type of questions that are asked in the exam.
NCERT Exemplar Class 10 Science Unit 4 Carbon and Its Compounds
NCERT Class 10 Science Unit 4 is for Carbon and Its Compounds. The type of questions that will be asked from NCERT Class 10 Science Unit 4 are displayed in the below provided NCERT Exemplar Class 10 Science Unit 4. With the help of it, candidates can prepare well for the examination.
Also Check: NCERT Solutions for Class 10 Science
Multiple Choice Questions
- Carbon exists in the atmosphere in the form of
(a) carbon monoxide only
(b) carbon monoxide in traces and carbon dioxide
(c) carbon dioxide only
(d) coal - Which of the following statements are usually correct for carbon compounds? These
(i) are good conductors of electricity
(ii) are poor conductors of electricity
(iii) have strong forces of attraction between their molecules
(iv) do not have strong forces of attraction between their molecules
(a) (i) and (iii)
(b) (ii) and (iii)
(c) (i) and (iv)
(d) (ii) and (iv) - A molecule of ammonia (NH3) has
(a) only single bonds
(b) only double bonds
(c) only triple bonds
(d) two double bonds and one single bond - Buckminsterfullerene is an allotropic form of
(a) phosphorus
(b) sulphur
(c) carbon
(d) tin - Which of the following are correct structural isomers of butane?
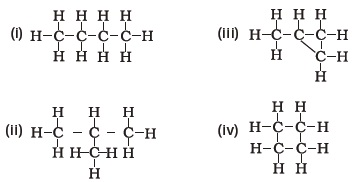
(a) (i) and (iii)
(b) (ii) and (iv)
(c) (i) and (ii)
(d) (iii) and (iv) 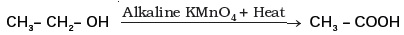
In the above given reaction, alkaline KMnO 4 acts as
(a) reducing agent
(b) oxidising agent
(c) catalyst
(d) dehydrating agent- Oils on treating with hydrogen in the presence of palladium or nickel catalyst form fats. This is an example of
(a) Addition reaction
(b) Substitution reaction
(c) Displacement reaction
(d) Oxidation reaction - In which of the following compounds, — OH is the functional group?
(a) Butanone
(b) Butanol
(c) Butanoic acid
(d) Butanal - The soap molecule has a
(a) hydrophilic head and a hydrophobic tail
(b) hydrophobic head and a hydrophilic tail
(c) hydrophobic head and a hydrophobic tail
(d) hydrophilic head and a hydrophilic tail - Which of the following is the correct representation of electron dot structure of nitrogen?
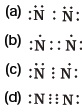
- Structural formula of ethyne is
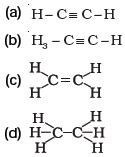
- Identify the unsaturated compounds from the following
(i) Propane
(ii) Propene
(iii) Propyne
(iv) Chloropropane
(a) (i) and (ii)
(b) (ii) and (iv)
(c) (iii) and (iv)
(d) (ii) and (iii) - Chlorine reacts with saturated hydrocarbons at room temperature in the
(a) absence of sunlight
(b) presence of sunlight
(c) presence of water
(d) presence of hydrochloric acid - In the soap micelles
(a) the ionic end of soap is on the surface of the cluster while the carbon chain is in the interior of the cluster.
(b) ionic end of soap is in the interior of the cluster and the carbon chain is out of the cluster.
(c) both ionic end and carbon chain are in the interior of the cluster
(d) both ionic end and carbon chain are on the exterior of the cluster - Pentane has the molecular formula C5H12. It has
(a) 5 covalent bonds
(b) 12 covalent bonds
(c) 16 covalent bonds
(d) 17 covalent bonds - Structural formula of benzene is

- Ethanol reacts with sodium and forms two products. These are
(a) sodium ethanoate and hydrogen
(b) sodium ethanoate and oxygen
(c) sodium ethoxide and hydrogen
(d) sodium ethoxide and oxygen - The correct structural formula of butanoic acid is
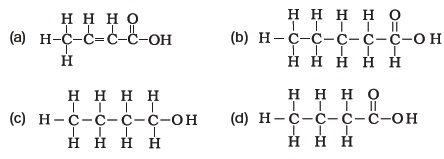
- Vinegar is a solution of
(a) 50% – 60% acetic acid in alcohol
(b) 5% – 8% acetic acid in alcohol
(c) 5% – 8% acetic acid in water
(d) 50% – 60% acetic acid in water - Mineral acids are stronger acids than carboxylic acids because
(i) mineral acids are completely ionised
(ii) carboxylic acids are completely ionised
(iii) mineral acids are partially ionised
(iv) carboxylic acids are partially ionised
(a) (i) and (iv)
(c) (i) and (ii)
(b) (ii) and (iii)
(d) (iii) and (iv) - Carbon forms four covalent bonds by sharing its four valence electrons with four univalent atoms, e.g. hydrogen. After the formation of four bonds, carbon attains the electronic configuration of
(a) helium
(b) neon
(c) argon
(d) krypton - The correct electron dot structure of a water molecule is
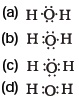
- Which of the following is not a straight chain hydrocarbon?
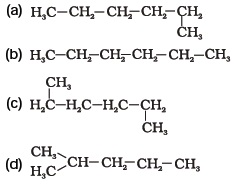
- Which among the following are unsaturated hydrocarbons?
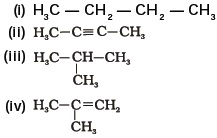
(a) (i) and (iii)
(b) (ii) and (iii)
(c) (ii) and (iv)
(d) (iii) and (iv) - Which of the following does not belong to the same homologous series?
(a) CH4
(b) C2H6
(c) C3H8
(d) C4H8 - The name of the compound CH 3 — CH 2 — CHO is
(a) Propanal
(b) Propanone
(c) Ethanol
(d) Ethanal - The heteroatoms present in
CH3 — CH2 — O — CH2 — CH2 Cl are
(i) oxygen
(ii) carbon
(iii) hydrogen
(iv) chlorine
(a) (i) and (ii)
(c) (iii) and (iv)
(b) (ii) and (iii)
(d) (i) and (iv) - Which of the following represents saponification reaction?
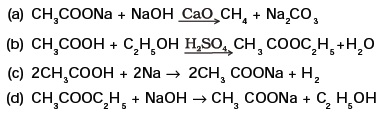
- The first member of alkyne homologous series is
(a) ethyne
(b) ethene
(c) propyne
(d) methane
Short Answer Type Questions
- Draw the electron dot structure of ethyne and also draw its structural formula
- Write the names of the following compounds
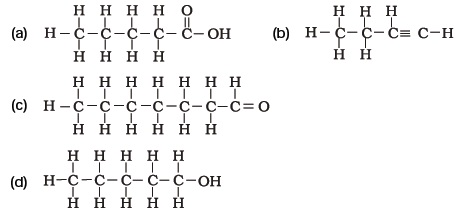
- Identify and name the functional groups present in the following compounds.
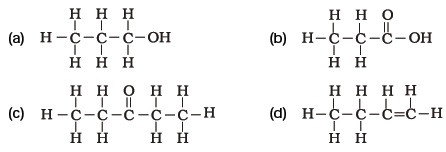
- A compound X is formed by the reaction of a carboxylic acid C2H4O2 and an alcohol in presence of a few drops of H2SO4. The alcohol on oxidation with alkaline KMnO4 followed by acidification gives the same carboxylic acid as used in this reaction. Give the names and structures of (a) carboxylic acid, (b) alcohol and (c) the compound X. Also write the reaction.
- Why detergents are better cleansing agents than soaps? Explain.
- Name the functional groups present in the following compounds
(a) CH3 CO CH2 CH2CH2CH3
(b) CH3CH2CH2COOH
(c) CH3CH2CH2CH2CHO
(d) CH3CH2OH - How is ethene prepared from ethanol? Give the reaction involved in it.
- Intake of small quantity of methanol can be lethal. Comment.
- A gas is evolved when ethanol reacts with sodium. Name the gas evolved and also write the balanced chemical equation of the reaction involved.
- Ethene is formed when ethanol at 443 K is heated with excess of concentrated sulphuric acid. What is the role of sulphuric acid in this reaction? Write the balanced chemical equation of this reaction.
- Carbon, Group (14) element in the Periodic Table, is known to form compounds with many elements.
Write an example of a compound formed with
(a) chlorine (Group 17 of Periodic Table)
(b) oxgygen (Group 16 of Periodic Table) - In electron dot structure, the valence shell electrons are represented by crosses or dots.
(a) The atomic number of chlorine is 17. Write its electronic configuration
(b) Draw the electron dot structure of chlorine molecule. - Catenation is the ability of an atom to form bonds with other atoms of the same element. It is exhibited by both carbon and silicon. Compare the ability of catenation of the two elements. Give reasons.
- Unsaturated hydrocarbons contain multiple bonds between the two C-atoms and show addition reactions. Give the test to distinguish ethane from ethene.
- Match the reactions given in Column (A) with the names given in column (B).
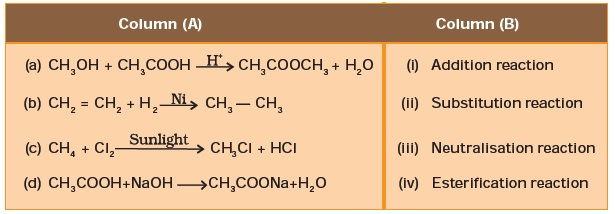
- Write the structural formulae of all the isomers of hexane.
- What is the role of metal or reagents written on arrows in the given chemical reactions?
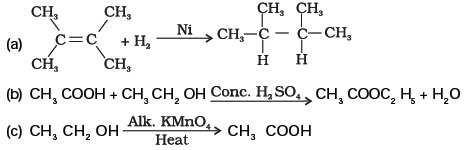
Long Answer Type Questions
- A salt X is formed and a gas is evolved when ethanoic acid reacts with sodium hydrogencarbonate. Name the salt X and the gas evolved. Describe an activity and draw the diagram of the apparatus to prove that the evolved gas is the one which you ave named. Also, write chemical equation of the reaction involved.
- (a) What are hydrocarbons? Give examples.
(b) Give the structural differences between saturated and unsaturated hydrocarbons with two examples each.
(c) What is a functional group? Give examples of four different functional groups. - Name the reaction which is commonly used in the conversion of vegetable oils to fats. Explain the reaction involved in detail.
- (a) Write the formula and draw electron dot structure of carbon tetrachloride.
(b) What is saponification? Write the reaction involved in this process. - Esters are sweet-smelling substances and are used in making perfumes. Suggest some activity and the reaction involved for the preparation of an ester with well labeled diagram.
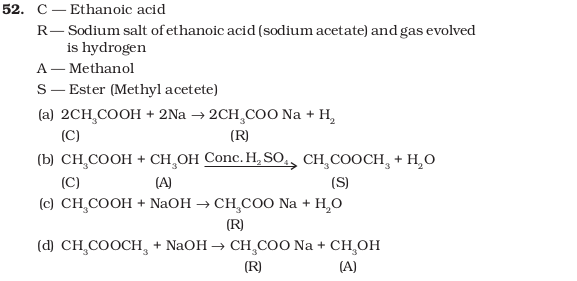
- A compound C (molecular formula, C2H4O2) reacts with Na – metal to form a compound R and evolves a gas which burns with a pop sound. Compound C on treatment with an alcohol A in presence of an acid forms a sweet smelling compound S (molecular formula, C3H6O2). On addition of NaOH to C, it also gives R and water. S on treatment with NaOH solution gives back R and A. Identify C, R, A, S and write down the reactions involved.
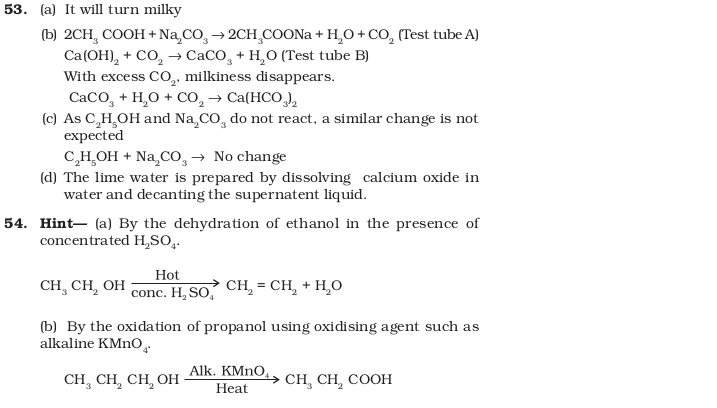
- Look at Figure 4.1 and answer the following questions
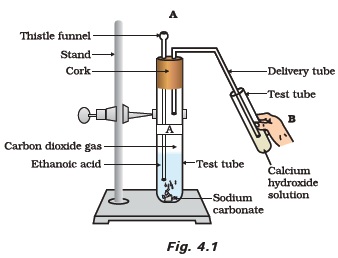
(a) What change would you observe in the calcium hydroxide solution taken in tube B?
(b) Write the reaction involved in test tubes A and B respectively.
(c) If ethanol is given instead of ethanoic acid, would you expect the same change?
(d) How can a solution of lime water be prepared in the laboratory? - How would you bring about the following conversions? Name the process and write the reaction involved. Fig. 4.1
(a) ethanol to ethene.
(b) propanol to propanoic acid.
Write the reactions. - Draw the possible isomers of the compound with molecular formula C3H6O and also give their electron dot structures.
- Explain the given reactions with the examples
(a) Hydrogenation reaction
(b) Oxidation reaction
(c) Substitution reaction
(d) Saponification reaction
(e) Combustion reaction - An organic compound A on heating with concentrated H2SO4 forms a compound B which on addition of one mole of hydrogen in presence of Ni forms a compound C. One mole of compound C on combustion forms two moles of CO3 and 3 moles of H2O. Identify the compounds A, B and C and write the chemical equations of the reactions involved.
| « Previous | Next » |
Answers
Multiple Choice Questions

Short Answer Questions





Long Answer Questions
To get study material, exam alerts and news, join our Whatsapp Channel.