Class 12 Chemistry Surface Chemistry – Get here the Notes for Class 12 Surface Chemistry. Candidates who are ambitious to qualify the Class 12 with good score can check this article for Notes. This is possible only when you have the best CBSE Class 12 Chemistry Notes, study material, and a smart preparation plan. CBSE 2019 Class 12th Exam is approaching and candidates will have to make the best use of the time available towards the last stage of your CBSE Class 12th Chemistry Preparation. To help you with that below we have provided the Notes of 12 Chemistry for topic Surface Chemistry.
- Class: 12th
- Subject: Chemistry
- Topic: Surface Chemistry
- Resource: Notes
CBSE Notes Class 12 Chemistry Surface Chemistry
Candidates who are pursuing in Class 12 are advised to revise the notes from this post. With the help of Notes, candidates can plan their Strategy for particular weaker section of the subject and study hard. So, go ahead and check the Important Notes for Class 12 Chemistry Surface Chemistry.
Surface Chemistry is the branch of chemistry which deals with the phenomenon that occurs on the surfaces or interfaces, such phenomenon includes corrosion. catalysis, crystallisation, etc
Adsorption
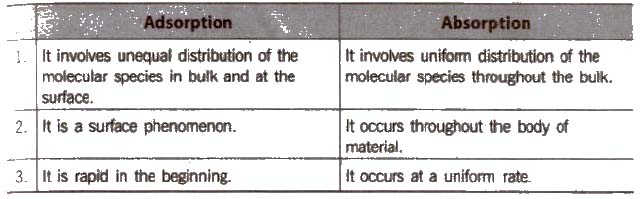
Due to unbalanced attraction forces, accumulation of molecular species at the surface rather than in the bulk of a solid or liquid is termed as adsorption. The molecular species accumulates at the surface is termed as adsorbate and the material on the surface of which the adsorption takes place is called adsorbent, e.g..
(i) O2, H2, C12, NB3 gases are adsorbed on the surface of charcoal.
(ii) Silica gels adsorb water molecules from air.
Charcoal, silica gel, metals such as Ni, Cu, Ag, Pt and colloids are some adsorbents.
Important Characteristics of Adsorption
1. It is specific and selective in nature.
2. Adsorption is spontaneous process, therefore change in free energy (ΔG)is negative.
ΔG= ΔH – TΔS,
For the negative value of ΔG,in a system, in which randomness decreases, ΔH must be negative. Hence, adsorption is always exothermic.
Adsorption of hydrogen over Pt is called occlusion.
Desorption
It is a process of removing an adsorbed substance from a surface on which it is adsorbed, is known as desorption.
Distinction between Adsorption and Absorption
Sorption
It is a process in which both adsorption and absorption take place simultaneously, the term sorption is simply used.
Positive and Negative Adsorption
When the concentration of the adsorbate is more on the surface of the adsorbent than in the bulk, it is called positive adsorption.
On the other hand, if the concentration of the adsorbate is less relative to its concentration in the bulk, it is called negative adsorption, e.g., when a dilute solution of KCl is shaken with blood charcoal, it shows negative adsorption.
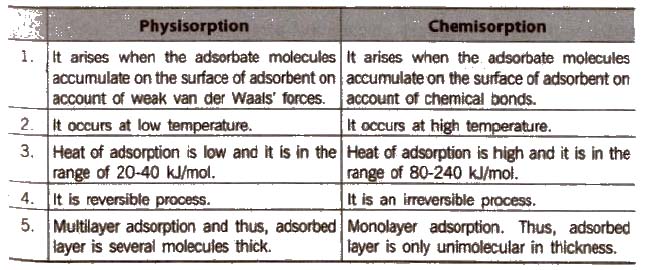
Distinction between Physisorption and Chemisorption
Factors Affecting Adsorption
(a) Nature of adsorbent Same gas may be adsorbed to different extents on different adsorbent.
(b) Surface area of the adsorbent Greater the surface area, greater is the extent of adsorption.
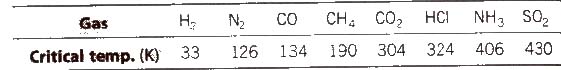
(c) Nature of the gas being adsorbed Greater is the critical temperature of a gas, greater are the van der Waals’ forces of attraction and thus, greater is the adsorption.
(d) Temperature Adsorption is an exothermic process involving the equilibrium :
Gas (adsorbate) + Solid (adsorbent) ⇔ Gas adsorbed on solid + Heat
Applying Le-Chatelier principle, increase of temperature decreases the adsorption and vice-versa.
(e) Pressure Adsorption increases with pressure at constant temperature. The effect is large if temperature is kept constant at low value.
(f) Activation of the solid adsorbent Activation means increasing the adsorbing power of the solid adsorbent. This can be done by subdividing the solid adsorbent or by removing the gases already adsorbed by passing superheated steam.
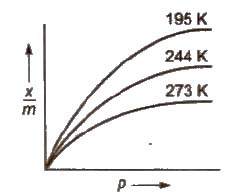
Adsorption Isotherms
It is the plot of the mass of gas adsorbed per gram of adsorbent (x / m) versus equilibrium pressure at constant temperature.
Freundlich Adsorption Isotherm
It gave an empirical relationship between the quantity of gas adsorbed by unit mass of solid adsorbent and pressure at a particular temperature. It can be expressed by the equation.
x / m = kp1/n …(i)
Where, x is the mass of the gas adsorbed on mass m of the adsorbent at pressure p, k and n are constants which depend on the nature of the adsorbent and the gas at a particular temperature.
At low pressure, n = 1, i.e., x / m = kp
At high pressure, n > 1, i.e., x / m = k (independent of p)
Taking logarithm of Eq. (i)
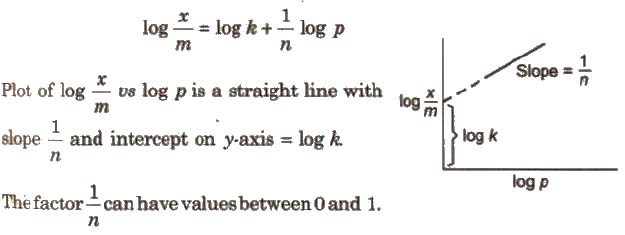
Freundlich Adsorption Equation for Solutions
x / m = kC1/n
where, C is the equilibrium concentration. On taking logarithm of the above equation, we have
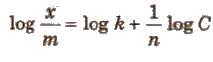
langmuir Adsorption Isotherm
According to Langmuir, the degree of adsorption is directly ProPOrtional to e, i.e., the fraction of surface area occupied.
x / m α θ = kθ
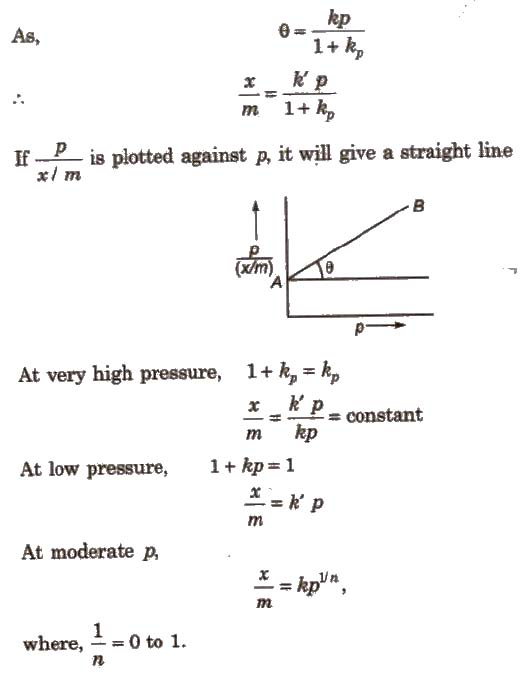
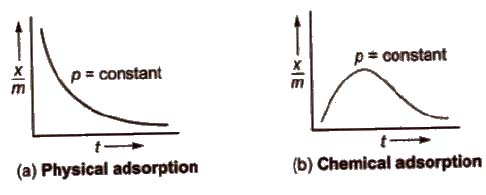
Adsorption Isobars
These are plots of x / m us temperature t at constant pressure. For physical and chemical adsorption, they are shown below.
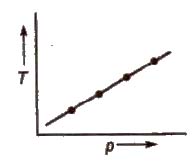
Adsorption Isostere
These are the plot of temperature versus pressure for a given amount of adsorption
Applications of Adsorption
- For production of high vacuum.
- Gas masks containing activated charcoal is used for breathing in coalmines. They adsorb poisonous gases.
- Silica and aluminium gels are used as adsorbents for controlling humidity.
- Removal of colouring matter from solutions.
- It is used in heterogeneous catalysis.
- In separation of inert gas.
- As adsorption indicators.
- In chromatographic analysis.
- Qualitative analysis, e.g., lake test for Al3+.
Catalysis
Catalyst is a chemical substance which can change the rate of reaction without being used up in that reaction and this process is known as catalysis
A catalyst may be positive (i.e., increases rate of reaction) or negative (i.e., decreases rate of reaction).
Types of Catalysis
(a) Homogeneous catalysis In this catalysis, and the catalyst reactants are in the same physical state [phase], e.g.,
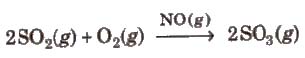
(b) Heterogeneous catalysis In heterogeneous catalysis, catalyst is present in a different phase than that of reactants, e.g.,
(c) Autocatalysis When one of the product of a reaction acts as catalyst, the process is called autocatalysis.
Characteristics of Catalysts
1 The catalyst remains unchanged in mass and chemical composition.
2. In case of reversible reactions, the catalyst does not influence the composition of reaction mixture at equilibrium. It only helps to attain the equilibrium quickly.
Promoters and Poisons
Promoters are chemical substances that enhance the activity of a catalyst while poisons decreases the activity of a catalyst
Adsorption Theory of Heterogeneous Catalysis
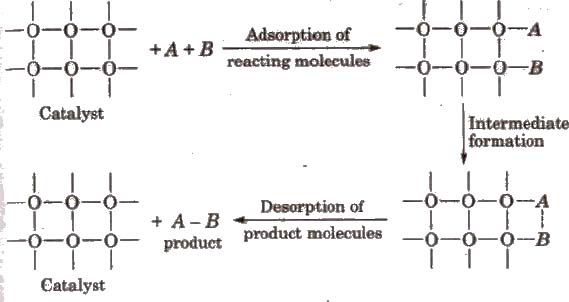
The mechanism involves five steps:
(i) Diffusion of reactants to the surface of the catalyst
(ii) Adsorption of reactant molecules on the surface of the catalyst.
(ill) Occurrence of chemical reaction on the catalyst’s surface through formation of an intermediate.
(iv) Desorption of reaction products from t he catalyst surface.
(v) Diffusion of reaction products away from the catalyst’s surface
Important Features of Solid Catalysts
(i) Activity The activity of a catalyst depends upon the strength of chemisorption to a large extent. The adsorption should be reasonably strong but not so strong that they become immobile and no space is available for other reactants to get adsorbed.
(ii) Selectivity The selectivity of a catalyst is its ability to direct a reaction to yield a particular product, e.g., starting with Hz and CO using different catalysts, we get different products.
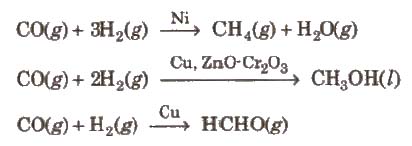
Shape–selective catalysis The catalytic reaction that depends upon the pore structure of the catalyst and the size of the reactant and product molecules is called shape-selective catalysiS. Cracking Isomerization of hydrocarbons in the presence of zeolites is an example of shape-selective catalysis.
An important zeolite catalyst used in the petroleum industry is ZSM-S.lt converts alcohols directly into gasoline.
Enzyme Catalysis
Enzymes are complex nitrogenous organic compounds which are Produced by living plants and animals. They are actually protein molecules of high molecular mass and form colloidal solutions in water.
They are also known as biochemical catalysis.
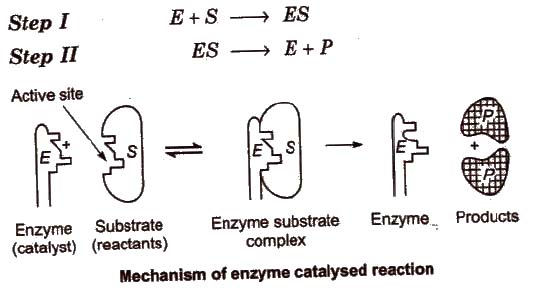
Mechanism of Enzyme Catalysis
Some examples of enzyme catalysed reactions are:
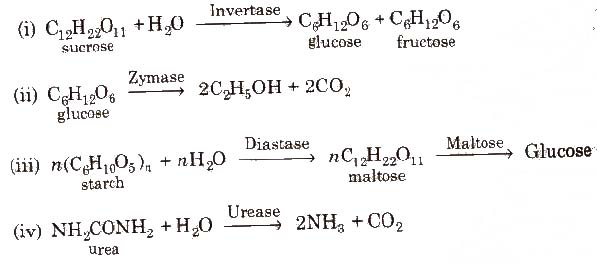
(Source of invertase, zymase and maltose is yeast and that of diastase is malt. Soybean is the source of urease.)
(v) In stomach, the pepsin enzyme converts proteins into peptides while in intestine, the pancreatic trypsin converts proteins into amino acids by hydrolysis.
(vi) Lactobacilli is used to convert milk into curd.
Characteristics of Enzyme Catalysis
- High efficiency One molecule of an enzyme may transform one million molecule of reactant per minute.
- Highly specific nature Each enzyme catalyst cannot catalyse more than one reaction.
- Optimum temperature Enzyme catalyst gives higher yield at optimum temperature i.e., at 298-310 K. Human body temperature, i.e., at being 310 K is suited for enzyme catalysed reactions.
- Optimum pH The rate of an enzyme catalysed reaction is maximum at optimum pH range 5 to 7.
- Activators Activators like ions such as Na+ ,Ca 2+, Mn2+ help in the activation of enzymes which cannot act on their own strength.
- Co-enzyme Co-enzymes are the substance having nature similar to the enzyme and their presence increases the enzyme activity. Mostly vitamins act as co-enzymes.
- Effect of Inhibitors Inhibitors slow down the rate of an enzymatic reaction. The use of many drugs is based on enzyme inhibition action of those drugs in the body.
Class 12 Key Points, Important Questions & Practice Papers
Hope these notes helped you in your schools exam preparation. Candidates can also check out the Key Points, Important Questions & Practice Papers for various Subjects for Class 12 in both Hindi and English language form the link below.
Class 12 NCERT Solutions
Candidates who are studying in Class 12 can also check Class 12 NCERT Solutions from here. This will help the candidates to know the solutions for all subjects covered in Class 12th. Candidates can click on the subject wise link to get the same. Class 12 Chapter-wise, detailed solutions to the questions of the NCERT textbooks are provided with the objective of helping students compare their answers with the sample answers.
Class 12 Mock Test / Practice
Mock test are the practice test or you can say the blue print of the main exam. Before appearing in the main examination, candidates must try mock test as it helps the students learn from their mistakes. With the help of Class 12 Mock Test / Practice, candidates can also get an idea about the pattern and marking scheme of that examination. For the sake of the candidates we are providing Class 12 Mock Test / Practice links below.
Class 12 Exemplar Questions
Exemplar Questions Class 12 is a very important resource for students preparing for the Examination. Here we have provided Exemplar Problems Solutions along with NCERT Exemplar Problems Class 12. Question from very important topics is covered by Exemplar Questions for Class 12.
Class 12 Chemistry Maths Notes Physics Notes Biology Notes
To get study material, exam alerts and news, join our Whatsapp Channel.


