Candidates can download NCERT Exemplar Class 12 Chemistry Chapter 6 from this page. The exemplar has been provided by the National Council of Educational Research & Training (NCERT) and the candidates can check it from below for free of cost. It contains objective, very short answer type, short answer type, and long answer type questions. Along with it, the answer for each question has also been provided. From the NCERT Exemplar Class 12 Chemistry Chapter 6, candidates can understand the level and type of questions that are asked in the exam.
NCERT Exemplar Class 12 Chemistry Chapter 6 General Principles and Processes of Isolation of Elements
NCERT Class 12 Chemistry Chapter 6 is for General Principles and Processes of Isolation of Elements. The type of questions that will be asked from NCERT Class 12 Chemistry Chapter 6 are displayed in the below provided NCERT Exemplar Class 12 Chemistry Chapter 6. With the help of it, candidates can prepare well for the examination.
Also Check: NCERT Solutions for Class 12 Chemistry
Multiple Choice Questions (Type I)
- In the extraction of chlorine by electrolysis of brine ____________.
(i) oxidation of Cl ion to chlorine gas occurs.
(ii) reduction of Cl ion to chlorine gas occurs.
(iii) For overall reaction ΔG has negative value.
(iv) a displacement reaction takes place. - When copper ore is mixed with silica, in a reverberatory furnace copper matte is produced. The copper matte contains ____________.
(i) sulphides of copper (II) and iron (II)
(ii) sulphides of copper (II) and iron (III)
(iii) sulphides of copper (I) and iron (II)
(iv) sulphides of copper (I) and iron (III) - Which of the following reactions is an example of autoreduction?
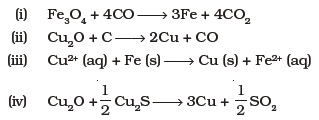
- A number of elements are available in earth’s crust but most abundant elements are ____________.
(i) Al and Fe
(ii) Al and Cu
(iii) Fe and Cu
(iv) Cu and Ag - Zone refining is based on the principle that ___________.
(i) impurities of low boiling metals can be separated by distillation.
(ii) impurities are more soluble in molten metal than in solid metal.
(iii) different components of a mixture are differently adsorbed on an adosrbent.
(iv) vapours of volatile compound can be decomposed in pure metal. - In the extraction of copper from its sulphide ore, the metal is formed by the reduction of Cu2O with
(i) FeS
(ii) CO
(iii) Cu2S
(iv) SO2 - Brine is electrolysed by using inert electrodes. The reaction at anode is ________.
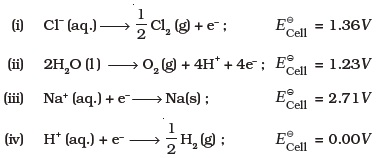
- In the metallurgy of aluminium ________________.
(i) Al3+ is oxidised to Al (s).
(ii) graphide anode is oxidised to carbon monoxide and carbon dioxide.
(iii) oxidation state of oxygen changes in the reaction at anode.
(iv) oxidation state of oxygen changes in the overall reaction involved in the process. - Electrolytic refining is used to purify which of the following metals?
(i) Cu and Zn
(ii) Ge and Si
(iii) Zr and Ti
(iv) Zn and Hg - Extraction of gold and silver involves leaching the metal with CN ion. The metal is recovered by ________________.
(i) displacement of metal by some other metal from the complex ion.
(ii) roasting of metal complex.
(iii) calcination followed by roasting.
(iv) thermal decomposition of metal complex.
Note : Answer the questions 11-13 on the basis of Fig. 6.1.
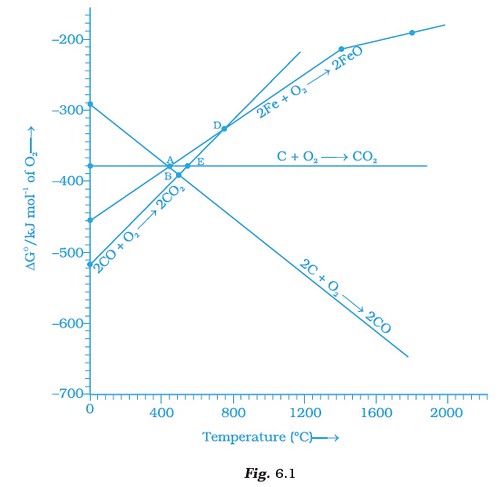
- Choose the correct option of temperature at which carbon reduces FeO to iron and produces CO.
(i) Below temperature at point A.
(ii) Approximately at the temperature corresponding to point A.
(iii) Above temperature at point A but below temperature at point D.
(iv) Above temperature at point A. - Below point ‘A’ FeO can ______________.
(i) be reduced by carbon monoxide only.
(ii) be reduced by both carbon monoxide and carbon.
(iii) be reduced by carbon only.
(iv) not be reduced by both carbon and carbon monoxide. - For the reduction of FeO at the temperature corresponding to point D, which of the following statements is correct?
(i) ΔG value for the overall reduction reaction with carbon monoxide is zero.
(ii) ΔG value for the overall reduction reaction with a mixture of 1 mol carbon and 1 mol oxygen is positive.
(iii) ΔG value for the overall reduction reaction with a mixture of 2 mol carbon and 1 mol oxygen will be positive.
(iv) ΔG value for the overall reduction reaction with carbon monoxide is negative.
Multiple Choice Questions (Type II)
Note : In the following questions two or more options may be correct.
- At the temperature corresponding to which of the points in Fig.6.1, FeO will be reduced to Fe by coupling the reaction 2FeO ⎯→ 2Fe + O2 with all of the following reactions?
(a) C + O2 ⎯→ CO2 (b) 2C + O2 ⎯→ 2CO and (c) 2CO + O2 ⎯→ 2 CO2
(i) Point A
(ii) Point B
(iii) Point D
(iv) Point E - Which of the following options are correct?
(i) Cast iron is obtained by remelting pig iron with scrap iron and coke using hot air blast.
(ii) In extraction of silver, silver is extracted as cationic complex.
(iii) Nickel is purified by zone refining.
(iv) Zr and Ti are purified by van Arkel method. - In the extraction of aluminium by Hall-Heroult process, purified Al2O3 is mixed with CaF2 to
(i) lower the melting point of Al2O3.
(ii) increase the conductivity of molten mixture.
(iii) reduce Al3+ into Al(s).
(iv) acts as catalyst. - Which of the following statements is correct about the role of substances added in the froth floation process?
(i) Collectors enhance the non-wettability of the mineral particles.
(ii) Collectors enhance the wettability of gangue particles.
(iii) By using depressants in the process two sulphide ores can be separated.
(iv) Froth stabilisers decrease wettability of gangue. - In the Froth Floatation process, zinc sulphide and lead sulphide can be separated by ______________.
(i) using collectors.
(ii) adjusting the proportion of oil to water.
(iii) using depressant.
(iv) using froth stabilisers. - Common impurities present in bauxite are ____________.
(i) CuO
(ii) ZnO
(iii) Fe2O3
(iv) SiO2 - Which of the following ores are concentrated by froth floation?
(i) Haematite
(ii) Galena
(iii) Copper pyrites
(iv) Magnetite - Which of the following reactions occur during calcination?
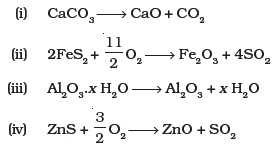
- For the metallurgical process of which of the ores calcined ore can be reduced by carbon?
(i) haematite
(ii) calamine
(iii) iron pyrites
(iv) sphalerite - The main reactions occurring in blast furnace during extraction of iron from haematite are ________.
(i) Fe2O3 + 3CO ⎯→ 2Fe + 3CO2
(ii) FeO + SiO2 ⎯→ FeSiO3
(iii) Fe2 O3 + 3C ⎯→ 2Fe + 3CO
(iv) CaO + SiO2 ⎯→ CaSiO3 - In which of the following method of purification, metal is converted to its volatile compound which is decomposed to give pure metal?
(i) heating with stream of carbon monoxide.
(ii) heating with iodine.
(iii) liquation.
(iv) distillation. - Which of the following statements are correct?
(i) A depressant prevents certain type of particle to come to the froth.
(ii) Copper matte contains Cu2S and ZnS.
(iii) The solidified copper obtained from reverberatory furnace has blistered appearance due to evolution of SO2 during the extraction.
(iv) Zinc can be extracted by self-reduction. - In the extraction of chlorine from brine _____________.
(i) ΔG for the overall reaction is negative.
(ii) ΔG for the overall reaction is positive.
(iii) E for overall reaction has negative value.
(iv) E for overall reaction has positive value.
Short Answer Type Questions
- Why is an external emf of more than 2.2V required for the extraction of Cl2from brine?
- At temperatures above 1073K coke can be used to reduce FeO to Fe. How can you justify this reduction with Ellingham diagram?
- Wrought iron is the purest form of iron. Write a reaction used for the preparation of wrought iron from cast iron. How can the impurities of sulphur, silicon and phosphorus be removed from cast iron?
- How is copper extracted from low grade copper ores?
- Write two basic requirements for refining of a metal by Mond process and by Van Arkel Method.
- Although carbon and hydrogen are better reducing agents but they are not used to reduce metallic oxides at high temperatures. Why?
- How do we separate two sulphide ores by Froth Floatation Method? Explain with an example.
- The purest form of iron is prepared by oxidising impurities from cast iron in a reverberatory furnace. Which iron ore is used to line the furnace? Explain by giving reaction.
- The mixture of compounds A and B is passed through a column of Al2O3 by using alcohol as eluant. Compound A is eluted in preference to compound B. Which of the compounds A or B, is more readily adsorbed on the column?
- Why is sulphide ore of copper heated in a furnace after mixing with silica?
- Why are sulphide ores converted to oxide before reduction?
- Which method is used for refining Zr and Ti? Explain with equation.
- What should be the considerations during the extraction of metals by electrochemical method?
- What is the role of flux in metallurgical processes?
- How are metals used as semiconductors refined? What is the principle of the method used?
- Write down the reactions taking place in Blast furnace related to the metallurgy of iron in the temperature range 500-800 K.
- Give two requirements for vapour phase refining.
- Write the chemical reactions involved in the extraction of gold by cyanide process. Also give the role of zinc in the extraction.
Matching Type Questions
Note : Match the items given in Column I and Column II in the following questions.
- Match the items of Column I with items of Column II and assign the correct code:
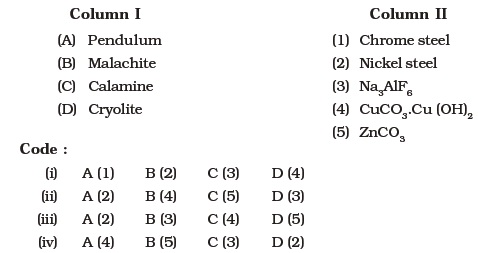
- Match the items of Column I with the items of Column II and assign the correct code :
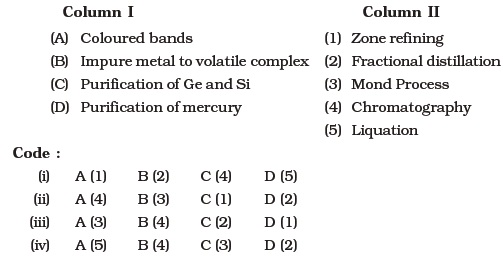
- Match items of Column I with the items of Column II and assign the correct code :
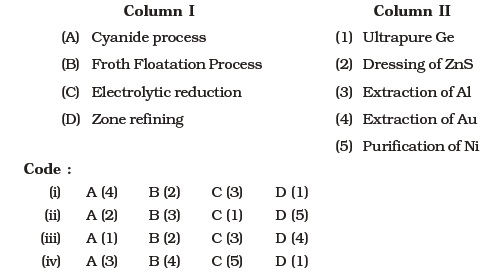
- Match the items of Column I with the items of Column II and assign the correct code :
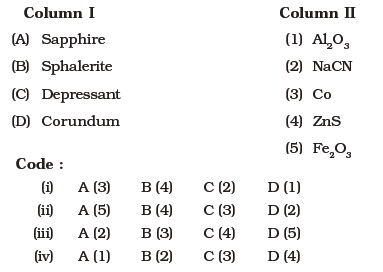
- Match the items of Column I with items of Column II and assign the correct code :
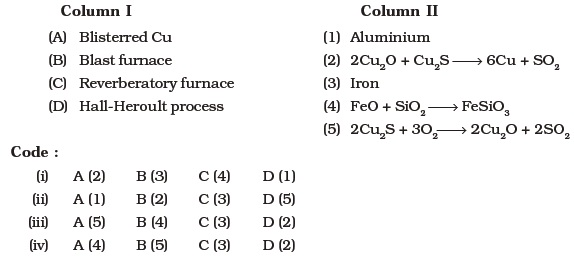
Assertion and Reason Type Questions
Note : In the following questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
(i) Both assertion and reason are true and reason is the correct explanation of assertion.
(ii) Both assertion and reason are true but reason is not the correct explanation of assertion.
(iii) Assertion is true but reason is false.
(iv) Assertion is false but reason is true.
(v) Assertion and reason both are wrong.
- Assertion : Nickel can be purified by Mond process.
Reason : Ni (CO)4 is a volatile compound which decomposes at 460K to give pure Ni. - Assertion : Zirconium can be purificed by Van Arkel method.
Reason : ZrI4 is volatile and decomposes at 1800K. - Assertion : Sulphide ores are concentrated by Froth Flotation method.
Reason : Cresols stabilise the froth in Froth Flotation method. - Assertion : Zone refining method is very useful for producing semiconductors.
Reason : Semiconductors are of high purity. - Assertion : Hydrometallurgy involves dissolving the ore in a suitable reagent followed by precipitation by a more electropositive metal.
Reason : Copper is extracted by hydrometallurgy.
Long Answer Type Questions
- Explain the following :
(a) CO2 is a better reducing agent below 710K whereas CO is a better reducing agent above 710K.
(b) Generally sulphide ores are converted into oxides before reduction.|
(c) Silica is added to the sulphide ore of copper in the reverberatory furnace.
(d) Carbon and hydrogen are not used as reducing agents at high temperatures.
(e) Vapour phase refining method is used for the purification of Ti.
| « Previous | Next » |
Answers






Chemistry Physics Maths Biology
To get study material, exam alerts and news, join our Whatsapp Channel.
