Class 12 Chemistry Haloalkanes and Haloarenes – Get here the Notes for Class 12 Haloalkanes and Haloarenes. Candidates who are ambitious to qualify the Class 12 with good score can check this article for Notes. This is possible only when you have the best CBSE Class 12 Chemistry Notes, study material, and a smart preparation plan. CBSE 2019 Class 12th Exam is approaching and candidates will have to make the best use of the time available towards the last stage of your CBSE Class 12th Chemistry Preparation. To help you with that, below we have provided the Notes of 12 Chemistry for topic Haloalkanes and Haloarenes.
- Class: 12th
- Subject: Chemistry
- Topic: Haloalkanes and Haloarenes
- Resource: Notes
CBSE Notes Class 12 Chemistry Haloalkanes and Haloarenes
Candidates who are pursuing in Class 12 are advised to revise the notes from this post. With the help of Notes, candidates can plan their Strategy for particular weaker section of the subject and study hard. So, go ahead and check the Important Notes for Class 12 Chemistry Haloalkanes and Haloarenes.
The replacement of hydrogen atom(s) in hydrocarbon, aliphatic or aromatic, by halogen atom(s) results in the formation of alkyl halide (haloalkane) and aryl halide (haloarene), respectively.
Classification of Halogen Derivatives
On the basis of number of halogen atoms present, halogen derivatives are classified as mono, di, tri, tetra, etc., halogen derivatives, e.g.,
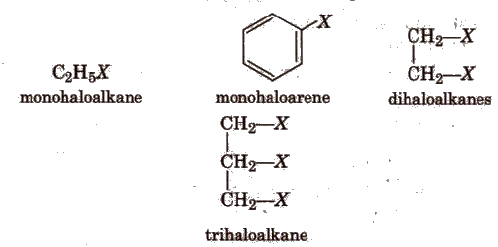
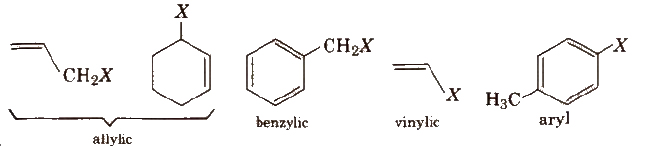
On the basis of the nature of the carbon to which halogen atom is attached, halogen derivatives are classified as 1°, 2°, 3°, allylic, benzylic, vinylic and aryl derivatives, e.g.,
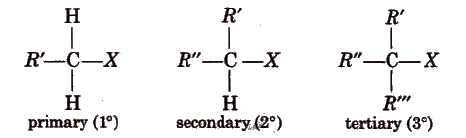
General Methods of Preparation of Haloalkanes
1. From Alcohols
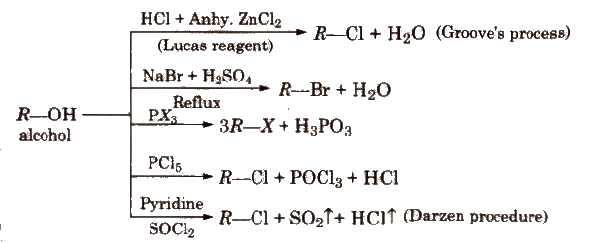
In Groove’s method, ZnC12 is used to weaken the C-OH bond. In case of 3° alcohols, ZnC12 is not required.
The reactivity order of halogen acids is HI > HBr > HCl.
Darzen procedure is the best method for preparing alkyl halides from alcohols since both the by products (SO2 and HCl) are gaseous and escape easily.
2. Free Radical Halogenation of Alkanes
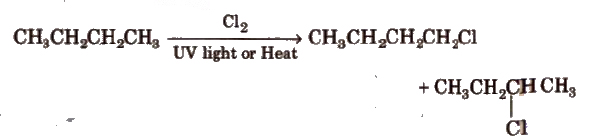
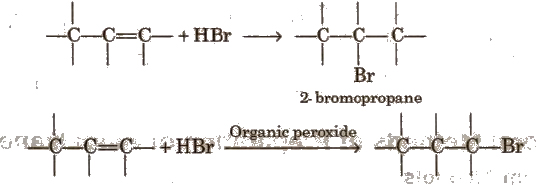
Addition of Hydrogen Halides on Alkenes
1. Finkelstein Reaction

2. Swarts Reaction
H3C – Br + AgF → H3C – F + AgBr
Hg2F2, COF2 and SbF3 can also be used as a reagent for Swarts reaction.
3. Hunsdiecker Reaction
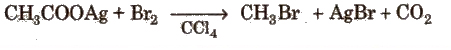
Physical Properties of Haloalkanes
1. Boiling point orders
- R – I > R – Br > R – CI > R – F
- CH3 – (CH2)2 – CH2Br > (CH3)2 CHCH2Br > (CH3)3CBr
- CH3CH2CH2 > CH3CH2X > CH3X
2. Bond strength of haloalkanes decreases as the size of the halogen atom increases. Thus, the order of bond strength is
CH3F > CR3Cl > CR3Br > CH3I
3. Dipole moment decreases as the electronegativity of the halogen decreases.
4. Haloalkanes though polar but are insoluble in water as they do not form hydrogen bonding with water.
5. Density order is
RI > RBr > RCl > RF (For the same alkyl group)
CH3I > C2H5I > C3H7I
Chemical Reactions of Haloalkanes
1. Nucleophilic Substitution Reactions (SN reactions)
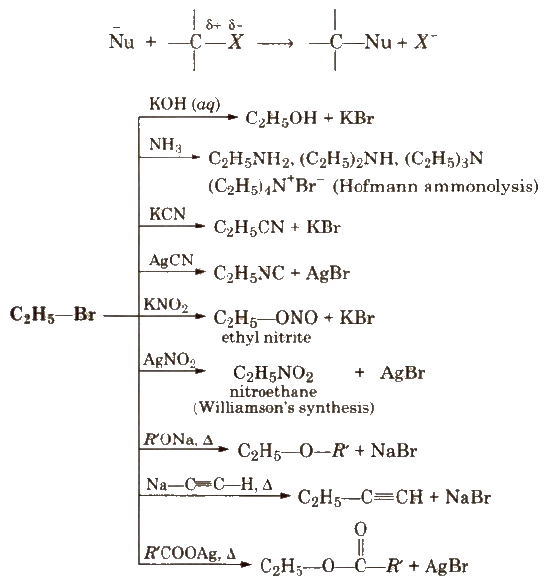
kCN is predominantly ionic and provides cyanide ions in solution, which is ambident nucleophile and bind with carbon side to form as the major product, while AgCN is covalent and form isocyanide as the major product.
Like KCN, KNO2 form R-ONO while AgNO2 produces R-NO2 as product. Vinyl chloride is less reactive towards nucleophilic substitution reactions due to resonance.
Nucleophilic substitution reactions are of two types
(a) SN1 type (Unimolecular nucleophilic reactions proceed in two steps:
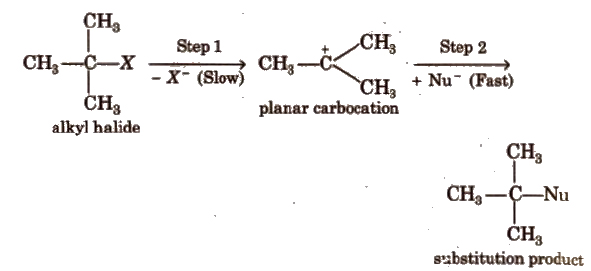
Rate, r = k [RX). It is a first order reaction.
Reactivity order of alkyl halide towards SN1 mechanism
3° > 2° > 1°
Polar solvents, low concentration of nucleophiles and weak nucleophiles favour SN1 mechanism.
In SN1 reactions, partial racemisation occurs due to the possibility of frontal as well as backside attack on planar carbocation.
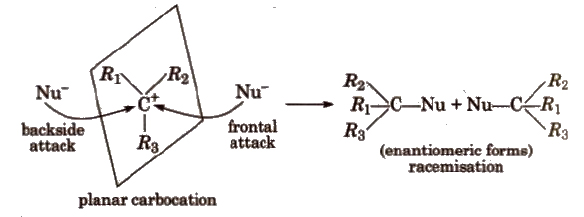
(b) SN2 type (Bimolecular nucleophilic substitution) These reactions proceed in one step and is a second order reaction with r = k[RX] [Nu].
During SN2 reaction, inversion of configuration occurs (Walden inversion) i.e., starting with dextrorotatory halide a laevo product is obtained and vice-versa, e.g.,
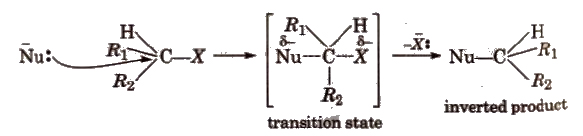
Reactivity of halides towards SN2 mechanism is
1° > 2° > 3°
Rate of reaction in SN2 mechanism depends on the strength of the attacking nucleophile. Strength of some common nucleophiles is
:CN– > : I– > : OR– > : OH– > CH3COO: > H2O > F–
Non-polar solvents, strong nucleophiles and high concentration of nucleophiles favour SN2 mechanism.
Relative rates of some alkyl halides in SN1 and SN2 reactions are in the order
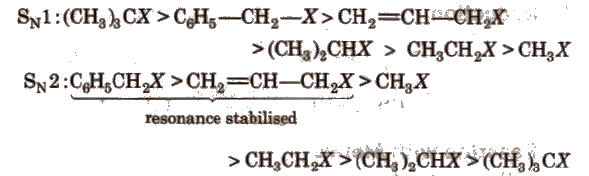
Resonating structure of benzyl carbocations are
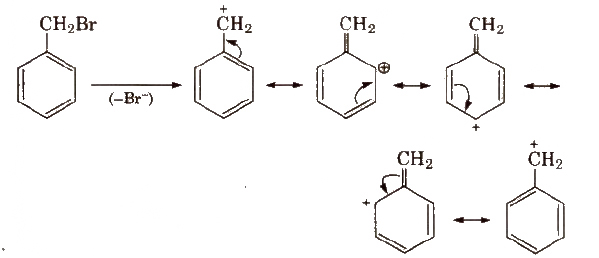
Relative reactivity of alkyl halides for same alkyl group is
RI > RBr > RCI > RF
2. Elimination Reactions
Dehydrohalogenation is a β – elimination reaction in which halogen is from α-carbon atom and the hydrogen from the α-carbon according to Saytzeff rule, e.g.,
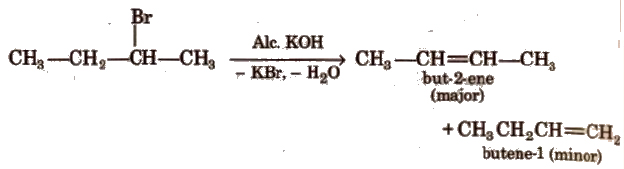
Ease of dehydrohalogenation among halides
3° > 2° > 1°
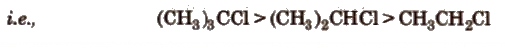
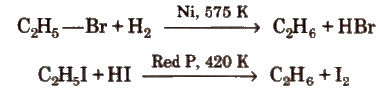
3. Reduction
4. Reaction with Metals
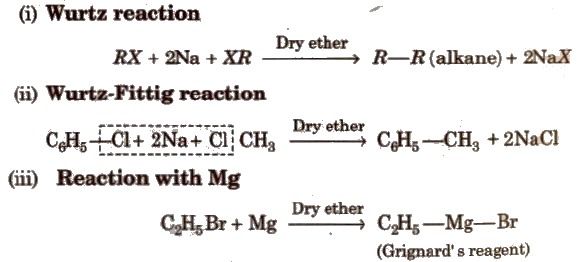
Grignard reagent is never isolated in the solid state as it explodes in dry state. So it is used as ethereal solution.
5. lsomerisation

General Methods of Preparation of Aryl Halides
1. By Halogenation of Aromatic Hydrocarbons

It is an electrophilic substitution reaction.
2. By Side Chain Halogenation
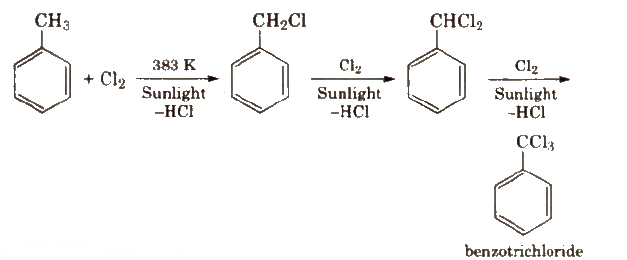
(It involves free radical mechanism.)
3. From Benzene Diazonium Salt
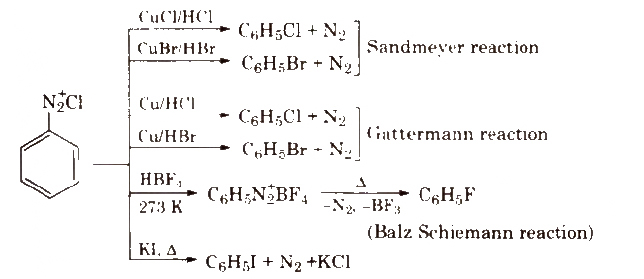
4. From Phenol
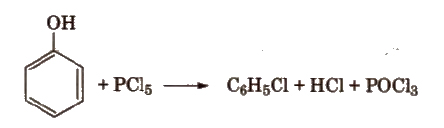
Physical Properties of Aryl Halides
1. Aryl halides are colourless liquids or colourless solids with characteristic odour.
2. Boiling point generally increases with increase in the size of aryl group or halogen atom. Boiling point order
Ar – I > Ar – Br > Ar – Cl > Ar – F
3. The melting point of p -isomer is more than 0- and m-isomer.
This is because of more symmetrical nature of p-isomer.
4. Due to resonance in chlorobenzene, C-CI bond is shorter and hence, its dipole moment is less than that ofcyclohexylchloride.
Chemical Properties of Aryl Halides
1. Nucleophilic Substitution Reaction
Aryl halides are less reactive towards nucleophilic substitution reaction. Their low reactivity is attributed due to the following reasons:
- Due to resonance, C-X bond has partial double bond character.
- Stabilisation of the molecule by delocalisation of electrons.
- (Instability of phenyl carbocation.
However, aryl halides having electron withdrawing groups (like – NO2, -SO3H, etc.) at ortho and para positions undergo nucleophilic substitution reaction easily.
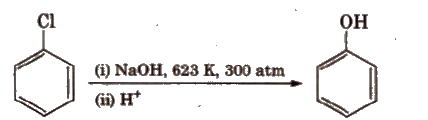
Presence of electron withdrawing group (-NO2) increases the reactivity.
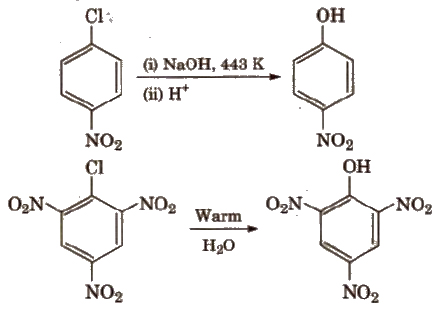
2. Electrophilic Substitution Reactions
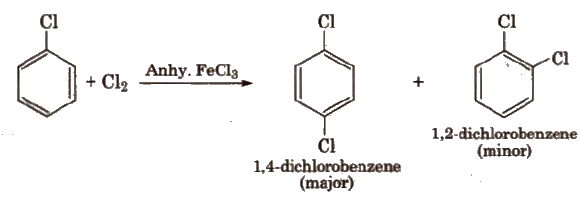
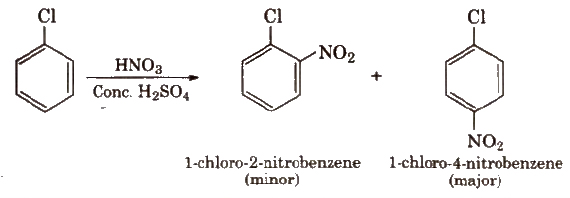
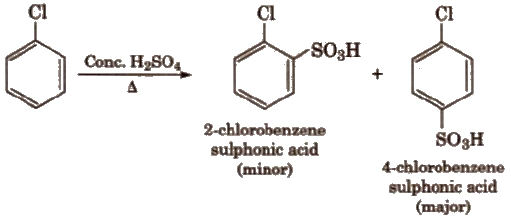
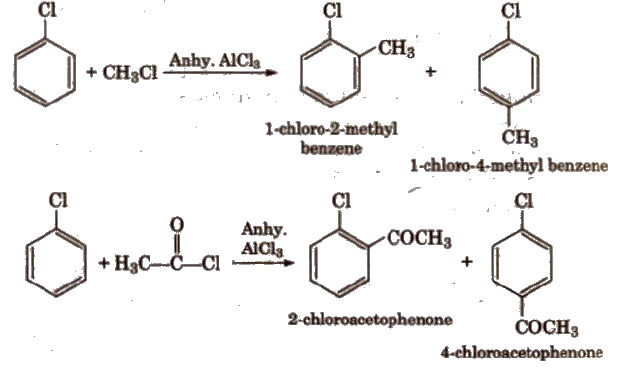
Halogens are deactivating but O, p-directing. Thus, chlorination, nitration, sulphonation and Friedel Craft’s reaction give a mixture of o- and P- chloro substituted derivatives.
(i) Halogenation
(ii) Nitration
(iii) Sulphonation
(iv) Friedel-Crafts reaction
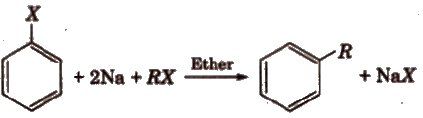
3. Reaction with Metals
(i) Wurtz Fittig reaction
(ii) Fitting reaction
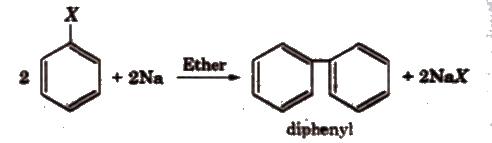
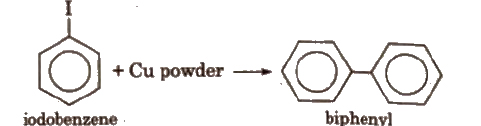
(iii) Ullmann reaction
Dlhalogen Derivatives
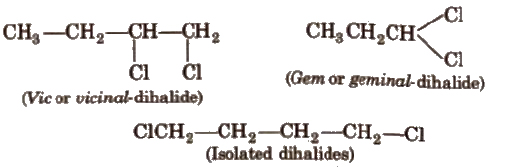
Dichloromethane (CH2Cl2) is widely used as a solvent, as a propellant in aerosols. Direct contact of dichloromethane in humans causes intense burning and milk redness of the skin.
Trihalogen Derivatives
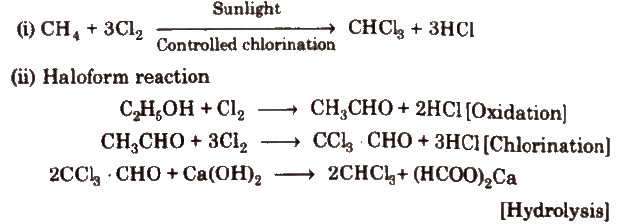
1. Chloroform [Trichloromethane, CHCl3]
Methods of preparation
Properties
1. Oxidation of CHCl3 gives poisonous gas phosgene (carbonyl chloride).

To avoid this oxidation CHCl3 iI .toreci in dark brown bottles and filled to the brim. 1% ethanol is added to chloroform which converts harmful phosgene gas into diethyl carbonate.
2. CHCl3 is widely used in the production of freon refrigerant R-22.
3. On nitration, it gives tear producing insecticide substance chloropicrin.
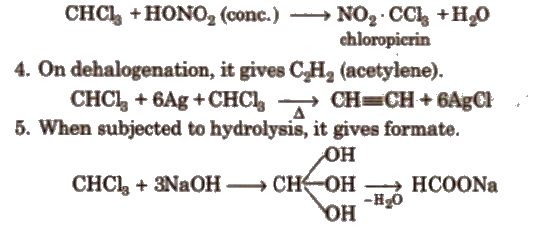
2. Iodoform (tri-iodornethane, CHl3)
Iodoform is prepared by iodoform reaction.
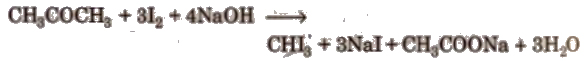
Compounds containing either CH3CO- or CH3CH(OH) group form yellow colour iodoform with I2 and NaOH.
Iodoform when comes in contact with organic matter, decomposes easily to free iodine, an antiseptic. Due to its objectionable smell, it has been replaced by other formulations containing iodine.
Polyhalogen Derivatives
1. Tetrachloromethane (Carbon Tetrachloride, CCl4 )
Preparation
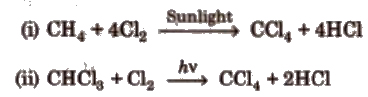
CCI4 is a colourless, non-inflammable, poisonous liquid, soluble in alcohol and ether.
Uses
Carbon tetrachloride is used
- as a solvent for oils, fats, resins
- in dry cleaning
- as fire extinguisher under the name ‘pyrene’.
2. Freons
The chlorofluorocarbon compounds of methane and ethane are collectively known as freons. These are usually produced for aerosol propellants, refrigeration and air conditioning purposes. Carbon tetra chloride when reacts with antimony trifluoride in the presence of SbCl5 as catalyst, dichlorofluromethane (freon) is obtained.
3. DDT (p, p’-Dichlorodiphenyltrichloroethane)
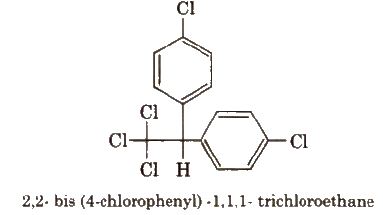
DDT is the first chlorinated organic insecticide. Its stability and fat solubility’is a great problem.
It is prepared from chloral and chlorobenzene in the presence of conc. H2SO4·
4. Perchloroethane (C2Cl6)
It is used as moth repellant and is also known as artificial camphor.
Class 12 Key Points, Important Questions & Practice Papers
Hope these notes helped you in your schools exam preparation. Candidates can also check out the Key Points, Important Questions & Practice Papers for various Subjects for Class 12 in both Hindi and English language form the link below.
Class 12 NCERT Solutions
Candidates who are studying in Class 12 can also check Class 12 NCERT Solutions from here. This will help the candidates to know the solutions for all subjects covered in Class 12th. Candidates can click on the subject wise link to get the same. Class 12 Chapter-wise, detailed solutions to the questions of the NCERT textbooks are provided with the objective of helping students compare their answers with the sample answers.
Class 12 Mock Test / Practice
Mock test are the practice test or you can say the blue print of the main exam. Before appearing in the main examination, candidates must try mock test as it helps the students learn from their mistakes. With the help of Class 12 Mock Test / Practice, candidates can also get an idea about the pattern and marking scheme of that examination. For the sake of the candidates we are providing Class 12 Mock Test / Practice links below.
Class 12 Exemplar Questions
Exemplar Questions Class 12 is a very important resource for students preparing for the Examination. Here we have provided Exemplar Problems Solutions along with NCERT Exemplar Problems Class 12. Question from very important topics is covered by Exemplar Questions for Class 12.
Class 12 Chemistry Maths Notes Physics Notes Biology Notes
To get study material, exam alerts and news, join our Whatsapp Channel.


