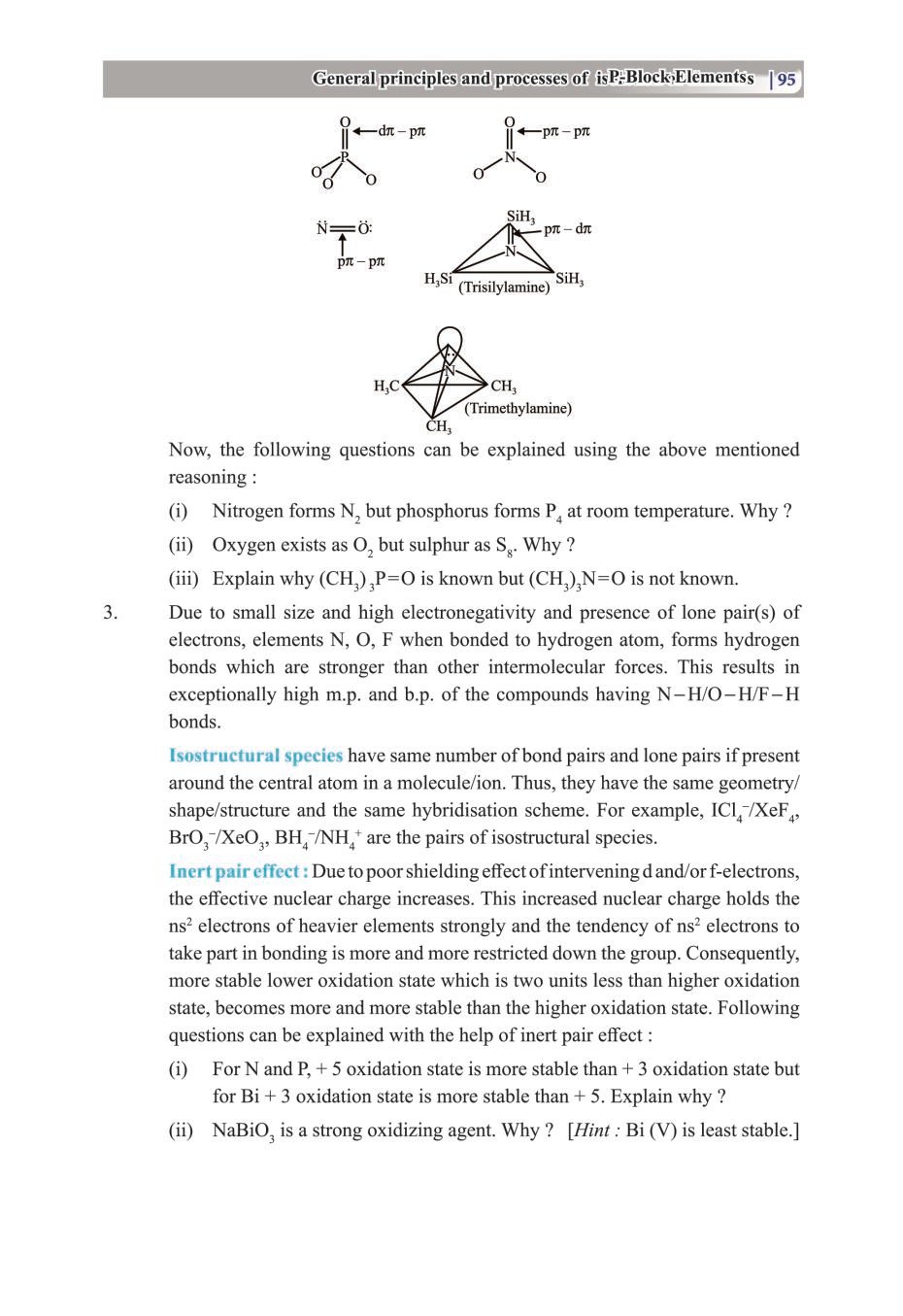
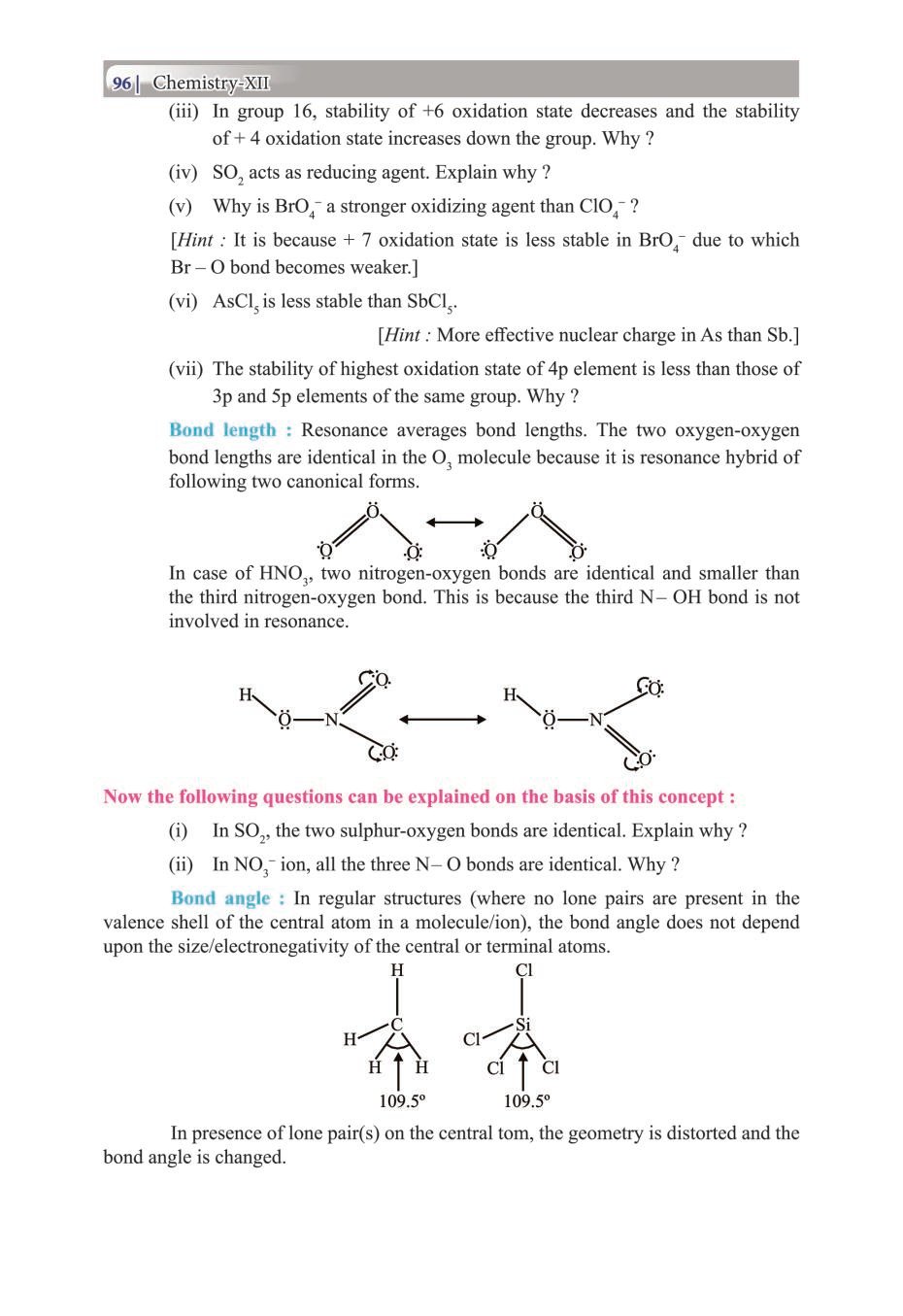
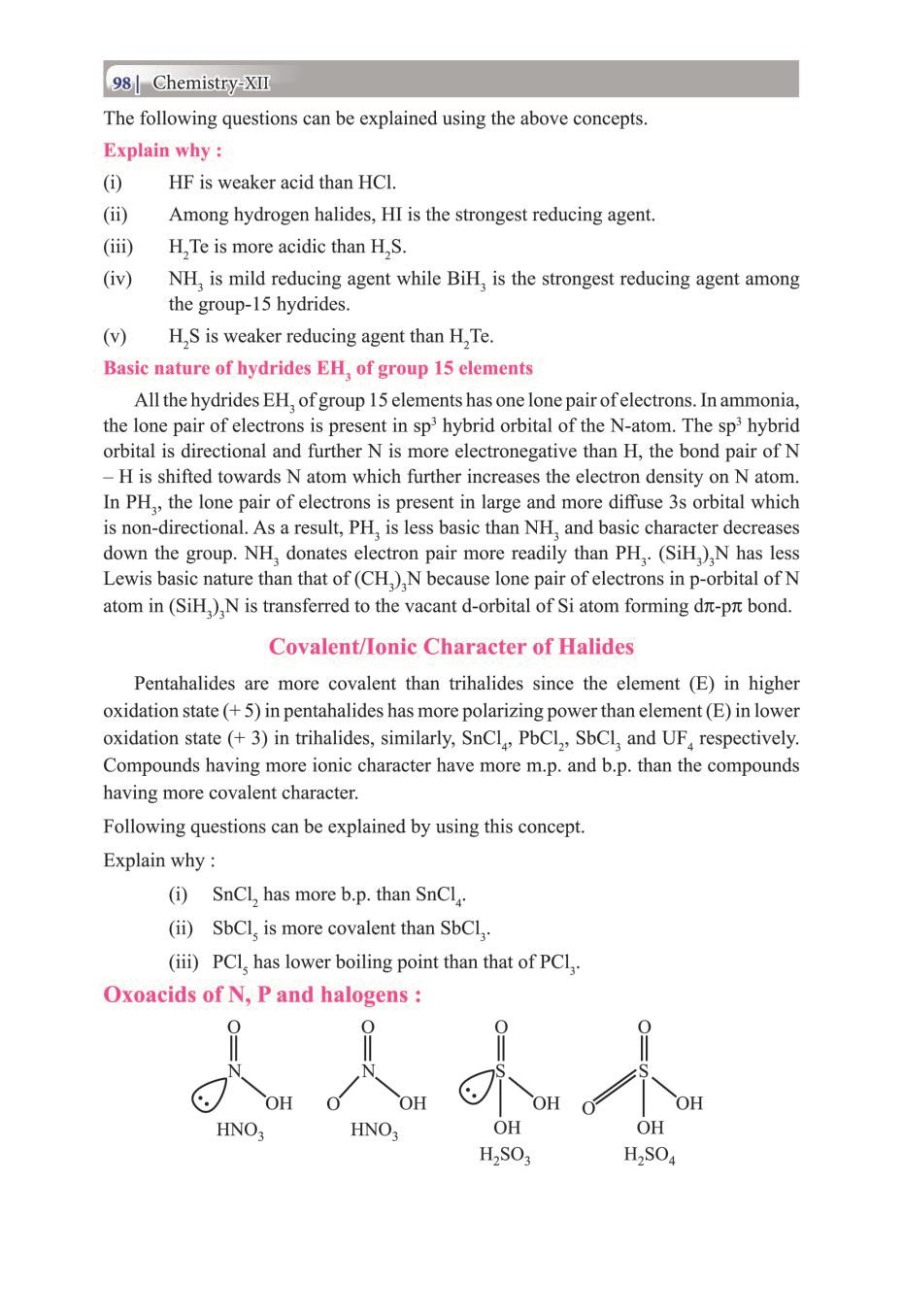
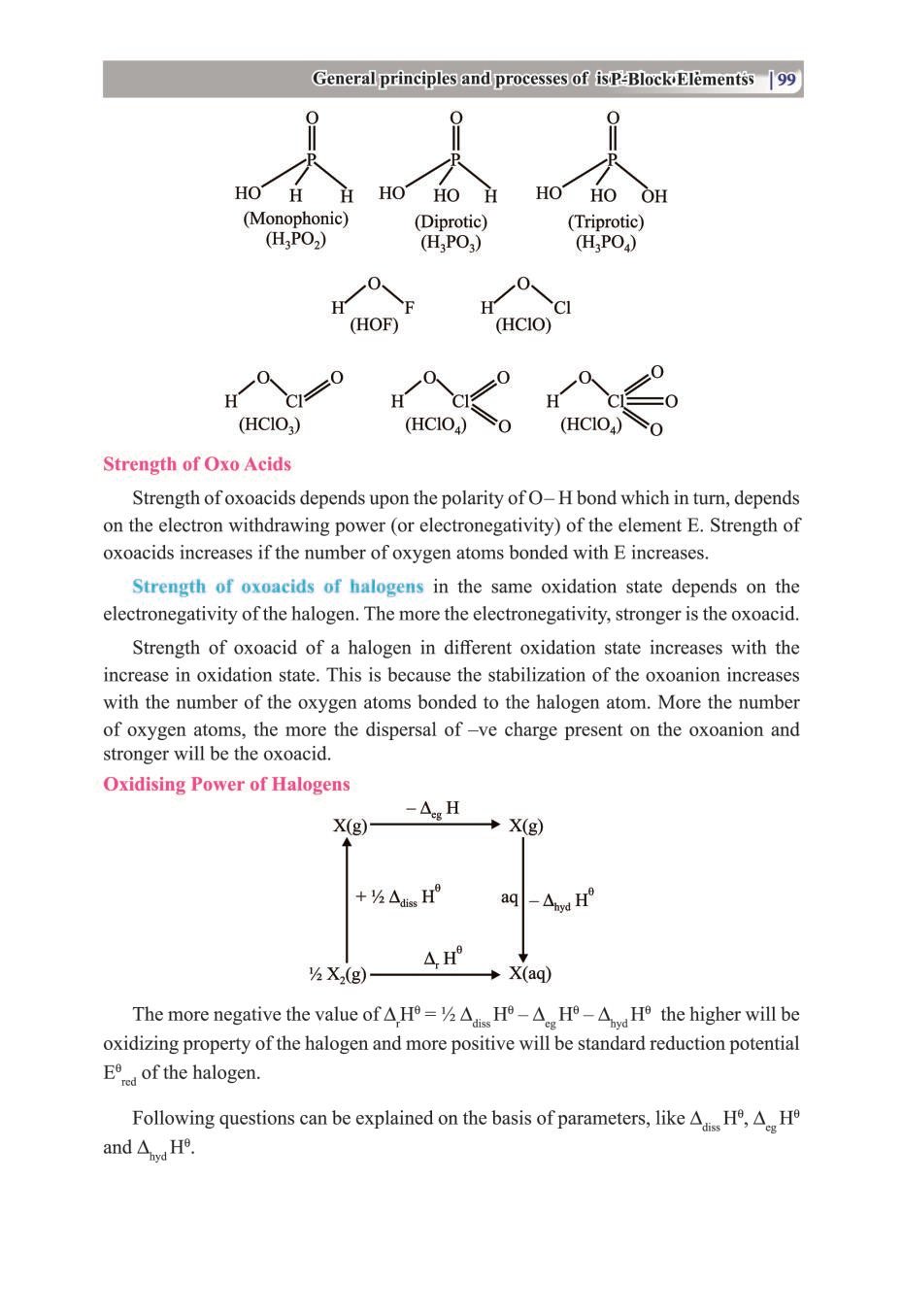
Class 12 Chemistry The P-Block Elements – Get here the Notes for Class 12 The P-Block Elements. Candidates who are ambitious to qualify the Class 12 with good score can check this article for Notes. This is possible only when you have the best CBSE Class 12 Chemistry Notes, study material, and a smart preparation plan. CBSE 2019 Class 12th Exam is approaching and candidates will have to make the best use of the time available towards the last stage of your CBSE Class 12th Chemistry Preparation. To help you with that, below we have provided the Notes of 12 Chemistry for topic The P-Block Elements.
- Class: 12th
- Subject: Chemistry
- Topic: The P-Block Elements
- Resource: Notes
CBSE Notes Class 12 Chemistry The P-Block Elements
Candidates who are pursuing in Class 12 are advised to revise the notes from this post. With the help of Notes, candidates can plan their Strategy for particular weaker section of the subject and study hard. So, go ahead and check the Important Notes for Class 12 Chemistry The P-Block Elements.
Class 12 Chemistry Notes for p-Block Elements View DownloadClass 12 Key Points, Important Questions & Practice Papers
Hope these notes helped you in your schools exam preparation. Candidates can also check out the Key Points, Important Questions & Practice Papers for various Subjects for Class 12 in both Hindi and English language form the link below.
Class 12 NCERT Solutions
Candidates who are studying in Class 12 can also check Class 12 NCERT Solutions from here. This will help the candidates to know the solutions for all subjects covered in Class 12th. Candidates can click on the subject wise link to get the same. Class 12 Chapter-wise, detailed solutions to the questions of the NCERT textbooks are provided with the objective of helping students compare their answers with the sample answers.
Class 12 Mock Test / Practice
Mock test are the practice test or you can say the blue print of the main exam. Before appearing in the main examination, candidates must try mock test as it helps the students learn from their mistakes. With the help of Class 12 Mock Test / Practice, candidates can also get an idea about the pattern and marking scheme of that examination. For the sake of the candidates we are providing Class 12 Mock Test / Practice links below.
Class 12 Exemplar Questions
Exemplar Questions Class 12 is a very important resource for students preparing for the Examination. Here we have provided Exemplar Problems Solutions along with NCERT Exemplar Problems Class 12. Question from very important topics is covered by Exemplar Questions for Class 12.
Class 12 Chemistry Maths Notes Physics Notes Biology Notes
To get study material, exam alerts and news, join our Whatsapp Channel.