Candidates can download NCERT Exemplar Class 12 Chemistry Chapter 11 from this page. The exemplar has been provided by the National Council of Educational Research & Training (NCERT) and the candidates can check it from below for free of cost. It contains objective, very short answer type, short answer type, and long answer type questions. Along with it, the answer for each question has also been provided. From the NCERT Exemplar Class 12 Chemistry Chapter 11, candidates can understand the level and type of questions that are asked in the exam.
NCERT Exemplar Class 12 Chemistry Chapter 11 Alcohols, Phenols and Ethers
NCERT Class 12 Chemistry Chapter 11 is for Alcohols, Phenols and Ethers. The type of questions that will be asked from NCERT Class 12 Chemistry Chapter 11 are displayed in the below provided NCERT Exemplar Class 12 Chemistry Chapter 11. With the help of it, candidates can prepare well for the examination.
Also Check: NCERT Solutions for Class 12 Chemistry
Multiple Choice Questions(Type-I)
- Monochlorination of toluene in sunlight followed by hydrolysis with aq. NaOH yields.
- o-Cresol
- m-Cresol
- 2, 4-Dihydroxytoluene
- Benzyl alcohol
- How many alcohols with molecular formula C4H10O are chiral in nature?
- 1
- 2
- 3
- 4
- What is the correct order of reactivity of alcohols in the following reaction?
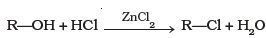
- 1° > 2° > 3°
- 1° < 2° > 3°
- 3° > 2° > 1°
- 3° > 1° > 2°
- CH3 CH3 OH2 can be converted into CH3CHO by ______________.
(i) catalytic hydrogenation
(ii) treatment with LiAlH4
(iii) treatment with pyridinium chlorochromate
(iv) treatment with KMnO4 - The process of converting alkyl halides into alcohols involves_____________.
(i) addition reaction
(ii) substitution reaction
(iii) dehydrohalogenation reaction
(iv) rearrangement reaction - Which of the following compounds is aromatic alcohol?
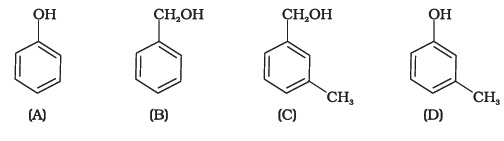
(i) A, B, C, D
(ii) A, D
(iii) B, C
(iv) A - Give IUPAC name of the compound given below.
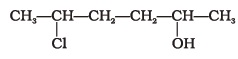
(i) 2-Chloro-5-hydroxyhexane
(ii) 2-Hydroxy-5-chlorohexane
(iii) 5-Chlorohexan-2-ol
(iv) 2-Chlorohexan-5-ol - IUPAC name of m-cresol is ___________.
(i) 3-methylphenol
(ii) 3-chlorophenol
(iii) 3-methoxyphenol
(iv) benzene-1,3-diol 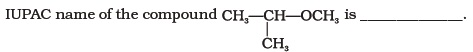
(i) 1-methoxy-1-methylethane
(ii) 2-methoxy-2-methylethane
(iii) 2-methoxypropane
(iv) isopropylmethyl ether- Which of the following species can act as the strongest base?
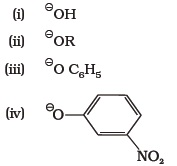
- Which of the following compounds will react with sodium hydroxide solution in water?
(i) C6H5OH
(ii) C6H5CH2OH
(iii) (CH3)3COH
(iv) C2H5OH - Phenol is less acidic than ______________.
(i) ethanol
(ii) o-nitrophenol
(iii) o-methylphenol
(iv) o-methoxyphenol - Which of the following is most acidic?
(i) Benzyl alcohol
(ii) Cyclohexanol
(iii) Phenol
(iv) m-Chlorophenol - Mark the correct order of decreasing acid strength of the following compounds.
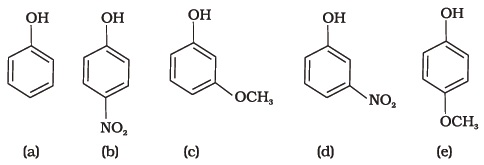
- e > d > b > a > c
- b > d > a > c > e
- d > e > c > b > a
- e > d > c > b > a
- Mark the correct increasing order of reactivity of the following compounds with HBr/HCl.
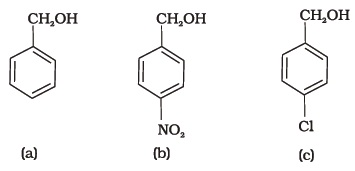
- Arrange the following compounds in increasing order of boiling point. Propan-1-ol, butan-1-ol, butan-2-ol, pentan-1-ol
- Propan-1-ol, butan-2-ol, butan-1-ol, pentan-1-ol
- Propan-1-ol, butan-1-ol, butan-2-ol, pentan-1-ol
- Pentan-1-ol, butan-2-ol, butan-1-ol, propan-1-ol
- Pentan-1-ol, butan-1-ol, butan-2-ol, propan-1-ol
Multiple Choice Questions(Type-II)
Note : In the following questions two or more options may be correct.
- Which of the following are used to convert RCHO into RCH2OH?
(i) H2/Pd
(ii) LiAlH4
(iii) NaBH4
(iv) Reaction with RMgX followed by hydrolysis - Which of the following reactions will yield phenol?
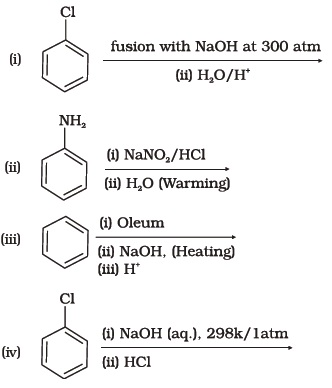
- Which of the following reagents can be used to oxidise primary alcohols to aldehydes?
- CrO3 in anhydrous medium.
- KMnO4 in acidic medium.
- Pyridinium chlorochromate.
- Heat in the presence of Cu at 573K.
- Phenol can be distinguished from ethanol by the reactions with _________.
(i) Br2/water
(ii) Na
(iii) Neutral FeCl3
(iv) All the above - Which of the following are benzylic alcohols?

Short Answer Type Questions
- What is the structure and IUPAC name of glycerol?
- Write the IUPAC name of the following compounds.
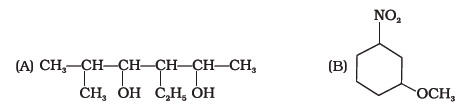
- Write the IUPAC name of the compound given below.
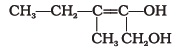
- Name the factors responsible for the solubility of alcohols in water.
- What is denatured alcohol?
- Suggest a reagent for the following conversion.
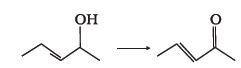
- Out of 2-chloroethanol and ethanol which is more acidic and why?
- Suggest a reagent for conversion of ethanol to ethanal.
- Suggest a reagent for conversion of ethanol to ethanoic acid.
- Out of o-nitrophenol and p-nitrophenol, which is more volatile? Explain.
- Out of o-nitrophenol and o-cresol which is more acidic?
- When phenol is treated with bromine water, white precipitate is obtained. Give the structure and the name of the compound formed.
- Arrange the following compounds in increasing order of acidity and give a suitable explanation. Phenol, o-nitrophenol, o-cresol
- Alcohols react with active metals e.g. Na, K etc. to give corresponding alkoxides. Write down the decreasing order of reactivity of sodium metal towards primary, secondary and tertiary alcohols.
- What happens when benzene diazonium chloride is heated with water?
- Arrange the following compounds in decreasing order of acidity. H2O, ROH, HC ≡ CH
- Name the enzymes and write the reactions involved in the preparation of ethanol from sucrose by fermentation.
- How can propan-2-one be converted into tert- butyl alcohol?
- Write the structures of the isomers of alcohols with molecular formula C4H10O. Which of these exhibits optical activity?
- Explain why is OH group in phenols more strongly held as compared to OH group in alcohols.
- Explain why nucleophilic substitution reactions are not very common in phenols.
- Preparation of alcohols from alkenes involves the electrophilic attack on alkene carbon atom. Explain its mechanism.
- Explain why is O==C==O nonpolar while R—O—R is polar.
- Why is the reactivity of all the three classes of alcohols with conc. HCl and ZnCl2 (Lucas reagent) different?
- Write steps to carry out the conversion of phenol to aspirin.
- Nitration is an example of aromatic electrophilic substitution and its rate depends upon the group already present in the benzene ring. Out of benzene and phenol, which one is more easily nitrated and why?
- In Kolbe’s reaction, instead of phenol, phenoxide ion is treated with carbon dioxide. Why?
- Dipole moment of phenol is smaller than that of methanol. Why?
- Ethers can be prepared by Williamson synthesis in which an alkyl halide is reacted with sodium alkoxide. Di-tert-butyl ether can’t be prepared by this method. Explain.
- Why is the C—O—H bond angle in alcohols slightly less than the tetrahedral angle whereas the C—O—C bond angle in ether is slightly greater?
- Explain why low molecular mass alcohols are soluble in water.
- Explain why p-nitrophenol is more acidic than phenol.
- Explain why alcohols and ethers of comparable molecular mass have different boiling points?
- The carbon-oxygen bond in phenol is slightly stronger than that in methanol. Why?
- Arrange water, ethanol and phenol in increasing order of acidity and give reason for your answer.
Matching Type Questions
Note : Match the items of Column I and Column II in the following questions.
- Match the structures of the compounds given in Column I with the name of the compounds given in Column II.
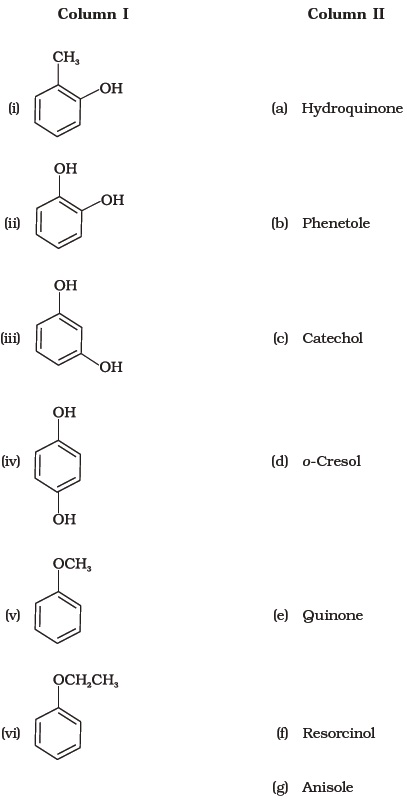
- Match the starting materials given in Column I with the products formed by these (Column II) in the reaction with HI.
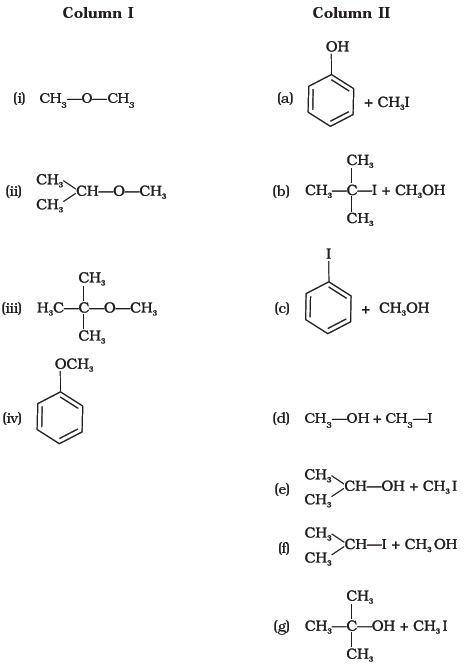
- Match the items of column I with items of column II.
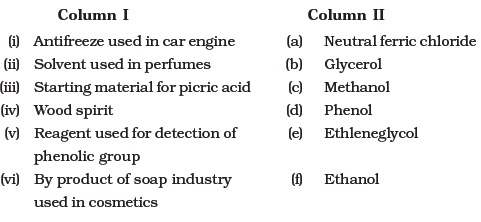
- Match the items of column I with items of column II.

Assertion and Reason Type Questions
Note : In the following questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
(i) Assertion and reason both are correct and reason is correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct statement but reason is wrong statement.
(iv) Assertion is wrong statement but reason is correct statement.
(v) Both assertion and reason are correct statements but reason is not correct explanation of assertion.
- Assertion : Addition reaction of water to but-1-ene in acidic medium yields butan-1-ol
Reason : Addition of water in acidic medium proceeds through the formation of primary carbocation. - Assertion : p-nitrophenol is more acidic than phenol.
Reason : Nitro group helps in the stabilisation of the phenoxide ion by dispersal of negative charge due to resonance. 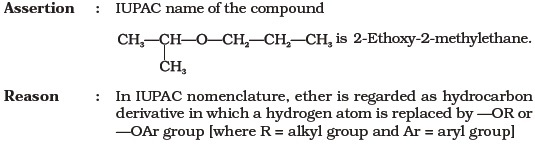
- Assertion : : Bond angle in ethers is slightly less than the tetrahedral angle.
Reason : : There is a repulsion between the two bulky (—R) groups. - Assertion : : Boiling points of alcohols and ethers are high.
Reason : They can form intermolecular hydrogen-bonding. - Assertion : Like bromination of benzene, bromination of phenol is also carried out in the presence of Lewis acid.
Reason : Lewis acid polarises the bromine molecule. - Assertion : o-Nitrophenol is less soluble in water than the m- and p-isomers.
Reason : m- and p- Nitrophenols exist as associated molecules. - Assertion : Ethanol is a weaker acid than phenol.
Reason : Sodium ethoxide may be prepared by the reaction of ethanol with aqueous NaOH. - Assertion :: Phenol forms 2, 4, 6 – tribromophenol on treatment with Br2 in carbon disulphide at 273K.
Reason : Bromine polarises in carbon disulphide. - Assertion : Phenols give o- and p-nitrophenol on nitration with conc. HNO3 and H2SO4mixture.
Reason : —OH group in phenol is o–, p– directing.
Long Answer Questions
- Write the mechanism of the reaction of HI with methoxybenzene.
- (a) Name the starting material used in the industrial preparation of phenol.
(b) Write complete reaction for the bromination of phenol in aqueous and non aqueous medium.
(c) Explain why Lewis acid is not required in bromination of phenol? - How can phenol be converted to aspirin?
- Explain a process in which a biocatalyst is used in industrial preparation of a compound known to you.
| « Previous | Next » |
Answers







Chemistry Physics Maths Biology
To get study material, exam alerts and news, join our Whatsapp Channel.


