Candidates can download NCERT Exemplar Class 12 Physics Chapter 12 from this page. The exemplar has been provided by the National Council of Educational Research & Training (NCERT) and the candidates can check it from below for free of cost. It contains objective, very short answer type, short answer type, and long answer type questions. Along with it, the answer for each question has also been provided. From the NCERT Exemplar Class 12 Physics Chapter 12, candidates can understand the level and type of questions that are asked in the exam.
NCERT Exemplar Class 12 Physics Chapter 12 Atoms
NCERT Class 12 Physics Chapter 12 is for Atoms. The type of questions that will be asked from NCERT Class 12 Physics Chapter 12 are displayed in the below provided NCERT Exemplar Class 12 Physics Chapter 12. With the help of it, candidates can prepare well for the examination.
Also Check: NCERT Solutions for Class 12 Physics
Multiple Choice Questions (MCQ I)
- Taking the Bohr radius as a0 = 53pm, the radius of Li++ ion in its ground state, on the basis of Bohr’s model, will be about
(a) 53 pm
(b) 27 pm
(c) 18 pm
(d) 13 pm - The binding energy of a H-atom, considering an electron moving around a fixed nuclei (proton),
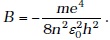 (m = electron mass). If one decides to work in a frame of reference where the electron is at rest, the proton would be moving around it. By similar arguments, the binding energy would be
(m = electron mass). If one decides to work in a frame of reference where the electron is at rest, the proton would be moving around it. By similar arguments, the binding energy would be 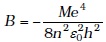 (M = proton mass)
(M = proton mass)
This last expression is not correct because
(a) n would not be integral.
(b) Bohr-quantisation applies only to electron
(c) the frame in which the electron is at rest is not inertial.
(d) the motion of the proton would not be in circular orbits, even approximately. - The simple Bohr model cannot be directly applied to calculate the energy levels of an atom with many electrons. This is because
(a) of the electrons not being subject to a central force.
(b) of the electrons colliding with each other
(c) of screening effects
(d) the force between the nucleus and an electron will no longer be given by Coulomb’s law. - For the ground state, the electron in the H-atom has an angular momentum = h , according to the simple Bohr model. Angular momentum is a vector and hence there will be infinitely many orbits with the vector pointing in all possible directions. In actuality, this is not true,
(a) because Bohr model gives incorrect values of angular momentum.
(b) because only one of these would have a minimum energy.
(c) angular momentum must be in the direction of spin of electron.
(d) because electrons go around only in horizontal orbits. - O2 molecule consists of two oxygen atoms. In the molecule, nuclear force between the nuclei of the two atoms
(a) is not important because nuclear forces are short-ranged.
(b) is as important as electrostatic force for binding the two atoms.
(c) cancels the repulsive electrostatic force between the nuclei.
(d) is not important because oxygen nucleus have equal number of neutrons and protons. - Two H atoms in the ground state collide inelastically. The maximum amount by which their combined kinetic energy is reduced is
(a) 10.20 eV
(b) 20.40 eV
(c) 13.6 eV
(d) 27.2 eV - A set of atoms in an excited state decays.
(a) in general to any of the states with lower energy.
(b) into a lower state only when excited by an external electric field.
(c) all together simultaneously into a lower state.
(d) to emit photons only when they collide.
Multiple Choice Questions (MCQ II)
- An ionised H-molecule consists of an electron and two protons. The protons are separated by a small distance of the order of angstrom. In the ground state,
(a) the electron would not move in circular orbits.
(b) the energy would be (2)4 times that of a H-atom.
(c) the electrons, orbit would go around the protons.
(d) the molecule will soon decay in a proton and a H-atom. - Consider aiming a beam of free electrons towards free protons. When they scatter, an electron and a proton cannot combine to produce a H-atom,
(a) because of energy conservation.
(b) without simultaneously releasing energy in the from of radiation.
(c) because of momentum conservation.
(d) because of angular momentum conservation. - The Bohr model for the spectra of a H-atom
(a) will not be applicable to hydrogen in the molecular from.
(b) will not be applicable as it is for a He-atom.
(c) is valid only at room temperature.
(d) predicts continuous as well as discrete spectral lines. - The Balmer series for the H-atom can be observed
(a) if we measure the frequencies of light emitted when an excited atom falls to the ground state.
(b) if we measure the frequencies of light emitted due to transitions between excited states and the first excited state.
(c) in any transition in a H-atom.
(d) as a sequence of frequencies with the higher frequencies getting closely packed. - Let
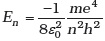 be the energy of the nth level of H-atom. If all the H-atoms are in the ground state and radiation of frequency (E2-E1)/h falls on it,
be the energy of the nth level of H-atom. If all the H-atoms are in the ground state and radiation of frequency (E2-E1)/h falls on it,
(a) it will not be absorbed at all
(b) some of atoms will move to the first excited state.
(c) all atoms will be excited to the n = 2 state.
(d) no atoms will make a transition to the n = 3 state. - The simple Bohr model is not applicable to He4 atom because
(a) He4 is an inert gas.
(b) He4 has neutrons in the nucleus.
(c) He4 has one more electron.
(d) electrons are not subject to central forces.
Very Short Answer Type Questions
- The mass of a H-atom is less than the sum of the masses of a proton and electron. Why is this?
- Imagine removing one electron from He4 and He3. Their energy levels, as worked out on the basis of Bohr model will be very close. Explain why.
- When an electron falls from a higher energy to a lower energy level, the difference in the energies appears in the form of electromagnetic radiation. Why cannot it be emitted as other forms of energy?
- Would the Bohr formula for the H-atom remain unchanged if proton had a charge (+4/3)e and electron a charge (−3/4)e , where e = 1.6 × 10-19C. Give reasons for your answer.
- Consider two different hydrogen atoms. The electron in each atom is in an excited state. Is it possible for the electrons to have different energies but the same orbital angular momentum according to the Bohr model?
Short Answer Type Questions
- Positronium is just like a H-atom with the proton replaced by the positively charged anti-particle of the electron (called the positron which is as massive as the electron). What would be the ground state energy of positronium?
- Assume that there is no repulsive force between the electrons in an atom but the force between positive and negative charges is given by Coulomb’s law as usual. Under such circumstances, calculate the ground state energy of a He-atom.
- Using Bohr model, calculate the electric current created by the electron when the H-atom is in the ground state.
- Show that the first few frequencies of light that is emitted when electrons fall to the nth level from levels higher than n, are approximate harmonics (i.e. in the ratio 1 : 2: 3…) when n >>1.
- What is the minimum energy that must be given to a H atom in ground state so that it can emit an Hγ line in Balmer series. If the angular momentum of the system is conserved, what would be the angular momentum of such Hγ photon?
Long Answer Type Questions
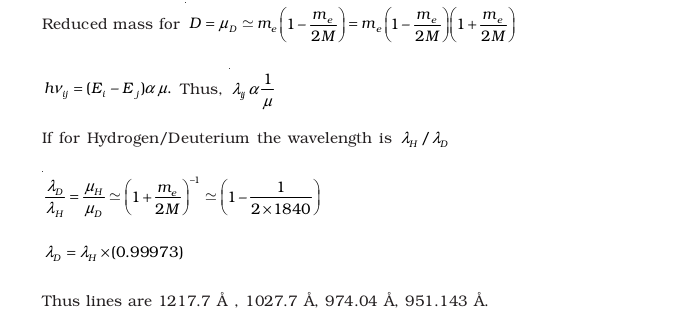
- The first four spectral lines in the Lyman series of a H-atom are λ = 1218 Å, 1028Å, 974.3 Å and 951.4Å. If instead of Hydrogen, we consider Deuterium, calculate the shift in the wavelength of these lines.
- Deuterium was discovered in 1932 by Harold Urey by measuring the small change in wavelength for a particular transition in 1H and 2H. This is because, the wavelength of transition depend to a certain extent on the nuclear mass. If nuclear motion is taken into account then the electrons and nucleus revolve around their common centre of mass. Such a system is equivalent to a single particle with a reduced mass μ, revolving around the nucleus at a distance equal to the electron-nucleus separation. Here μ = meM/(me + M) where M is the nuclear mass and me is the electronic mass. Estimate the percentage difference in wavelength for the 1st line of the Lyman series in 1H and 2H. (Mass of 1H nucleus is 1.6725 × 10-27 kg, Mass of 2H nucleus is 3.3374 × 10-27 kg, Mass of electron = 9.109 × 10-31 kg.)
- If a proton had a radius R and the charge was uniformly distributed, calculate using Bohr theory, the ground state energy of a H-atom when (i) R = 0.1Å, and (ii) R = 10 Å.
- In the Auger process an atom makes a transition to a lower state without emitting a photon. The excess energy is transferred to an outer electron which may be ejected by the atom. (This is called an Auger electron). Assuming the nucleus to be massive, calculate the kinetic energy of an n = 4 Auger electron emitted by Chromium by absorbing the energy from a n = 2 to n = 1 transition.
- The inverse square law in electrostatics is
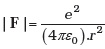 for the force between an electron and a proton. The (1/r) dependence of |F| can be understood in quantum theory as being due to the fact that the ‘particle’ of light (photon) is massless. If photons had a mass mp, force would be modified to
for the force between an electron and a proton. The (1/r) dependence of |F| can be understood in quantum theory as being due to the fact that the ‘particle’ of light (photon) is massless. If photons had a mass mp, force would be modified to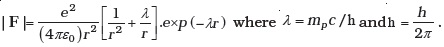
Estimate the change in the ground state energy of a H-atom if mp were 10–6 times the mass of an electron. - The Bohr model for the H-atom relies on the Coulomb’s law of electrostatics. Coulomb’s law has not directly been verified for very short distances of the order of angstroms. Supposing Coulomb’s law between two opposite charge + q1, –q2 is modified to
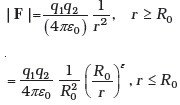
Calculate in such a case, the ground state energy of a H-atom, if ε = 0.1, R0 = 1Å.
| « Previous | Next » |
Answers to Multiple Choice Questions














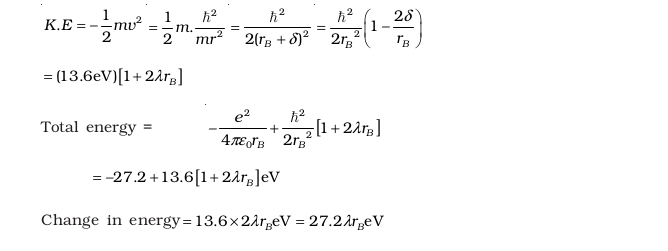




Physics Chemistry Maths Biology
To get study material, exam alerts and news, join our Whatsapp Channel.


